Preparation method of stable calcium dobesilate
A calcium dobesilate, stable technology, applied in the field of medicine, can solve the problems of reducing drug efficacy, adverse reactions, and easy staining on the appearance, and achieve the effect of improving stability, ensuring effectiveness and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
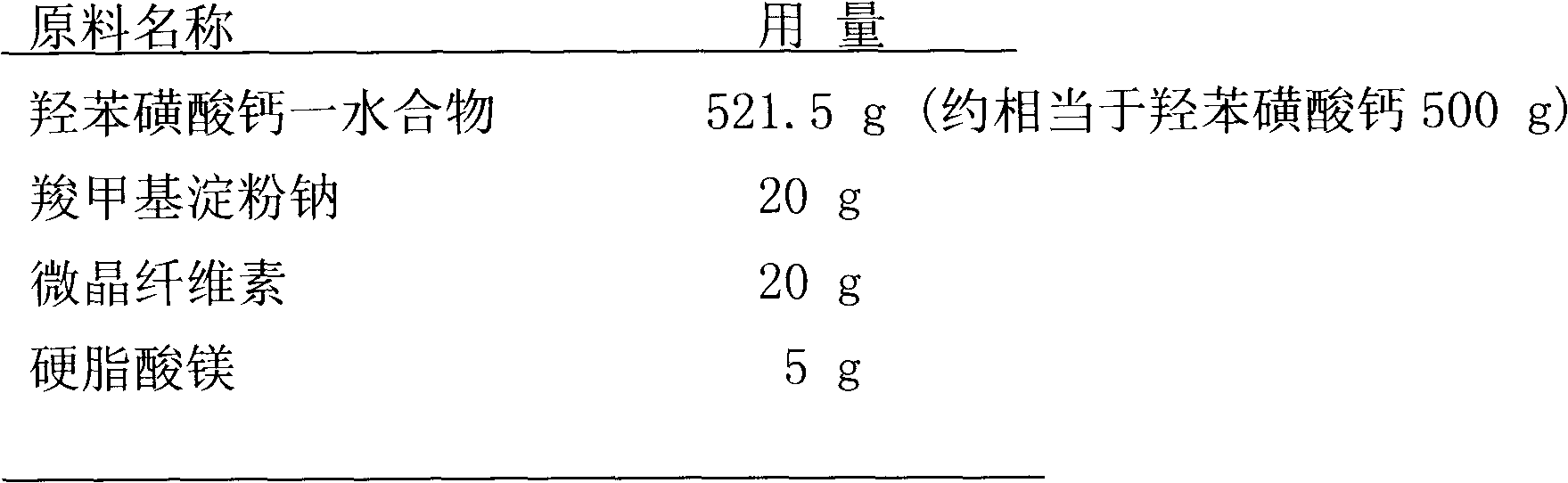
[0037] Preparation of calcium dobesilate tablets by dry method and direct compression
[0038] prescription
[0039]
[0040]
[0041] Preparation Process
[0042] The raw and auxiliary materials are dried (when the moisture content is less than 2%), weighed according to the prescription, respectively passed through a 100 mesh sieve, mixed evenly, passed through an 80 mesh sieve 3 times, directly added to the hopper, the environmental humidity is not more than 65% , Use a flat concave head with a diameter of 9-11mm to punch the tablet, and control the radial pressure of the compressed tablet to 6-10kg.
Embodiment 2
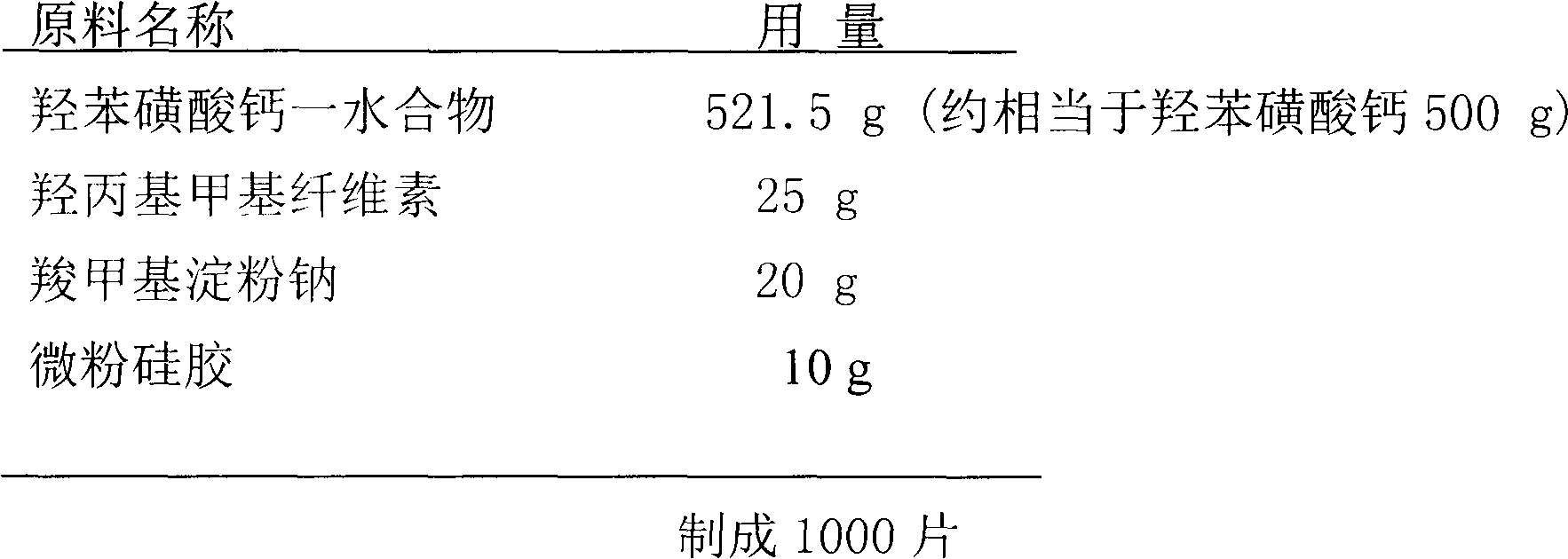
[0044] Preparation of calcium dobesilate film-coated tablets by dry compression and granulation
[0045] prescription:
[0046]
[0047] Preparation Process:
[0048] Weigh the raw and auxiliary materials (except micro-powder silica gel) according to the prescribed amount, respectively pass through a 100-mesh sieve, mix well, pass through an 80-mesh sieve 3 times, and directly add to the hopper. The ambient humidity is not more than 65%, and a flat concave head with a diameter of more than 12mm is used. For punching tablets, the radial pressure of the compressed tablets is controlled at 3~5kg.
[0049] Crush the pressed large tablets into uniform particles, pass through an 18-mesh sieve, add the prescription amount of fine silica gel, mix evenly, add to the hopper, adjust the pressure to 6-10kg, and press the tablets with a punch with a diameter of 9-11mm.
[0050] The compressed tablets are made into a coating material with an anhydrous ethanol solution of polyvinyl dialdehyde diethyl...
Embodiment 3
[0052] Preparation of Calcium Dobesilate Capsules by Dry Compression and Granulation
[0053] prescription:
[0054]
[0055]
[0056] Preparation Process:
[0057] Weigh the raw and auxiliary materials (except magnesium stearate) according to the prescribed amount, respectively pass through a 100-mesh sieve, mix well, pass through an 80-mesh sieve 3 times, and directly add to the hopper. The ambient humidity is not more than 65%. Use a flat with a diameter of more than 12mm. With concave head punching tablets, the radial pressure of the compressed tablets is controlled at 3~5kg.
[0058] The crushed large tablets are crushed into uniform particles, passed through an 18-mesh sieve, and the prescription amount of finely powdered silica gel is added, and after mixing uniformly, the capsules are filled to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com