Pharmaceutical composition for treating diabetic feet caused by diabetes and acro-skin lesion and preparation method of pharmaceutical composition
A technology for diabetic foot and skin lesions, applied in the field of pharmaceutical compositions and their preparation, can solve the problems of difficult healing of surgical wounds, peripheral nerve damage, and microcirculation disorders, and achieves inhibition of platelet aggregation, promotion of nerve cell differentiation, improvement of The effect of microcirculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
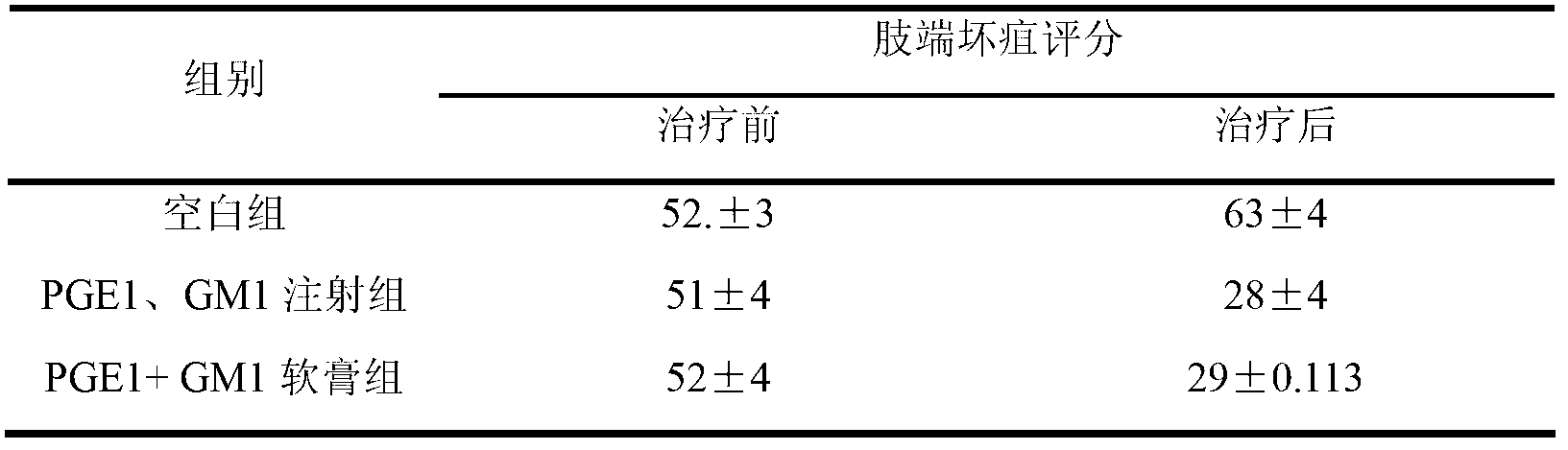
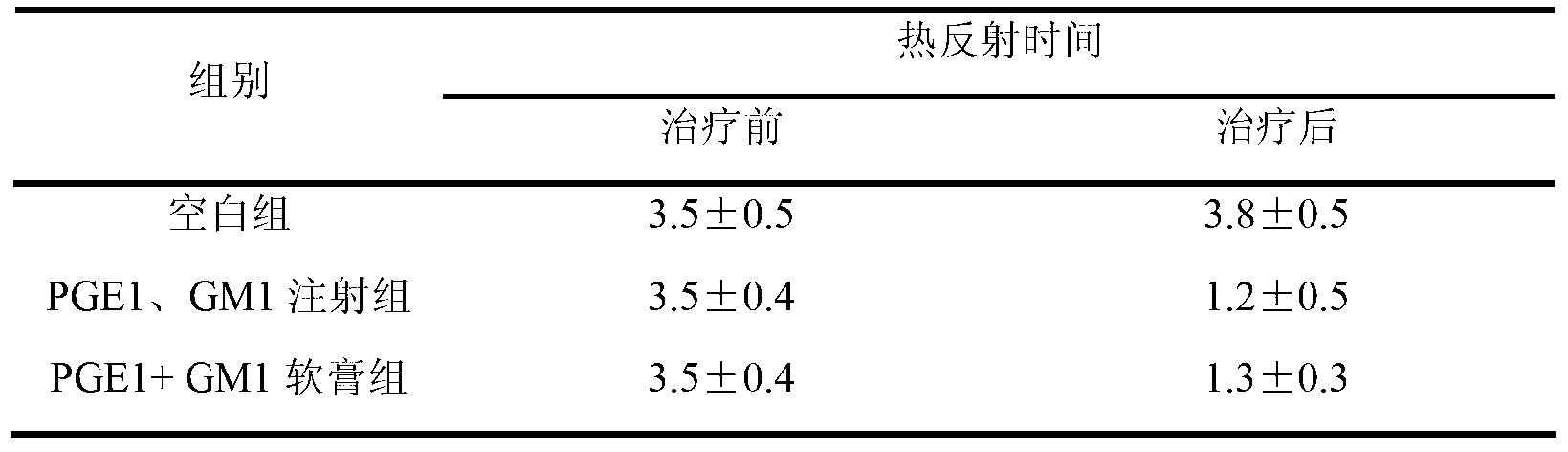
Examples
Embodiment 1
[0022] Element:
[0023] PGE1: 20mg, GM1: 2g;
[0024] HP-β-CD: 1.5g, petrolatum: 200g, polyacrylic acid: 100g, cetyl alcohol: 50g, triethanolamine: 3ml, Tween-80: 100g, glycerin: 50g, propylene glycol: 100g, benzoic acid: 0.5g, Absolute ethanol: 3ml, make up the balance with purified water.
[0025] Preparation method: Take PGE1 according to the prescription, add absolute ethanol to dissolve; take HP-β-CD and add purified water to dissolve, mix the two solutions, ultrasonically treat for 10 minutes, and freeze-dry. The obtained PGE1 inclusion compound is placed in a desiccator for later use; Polyacrylic acid, cetyl alcohol, heated to 75 ℃ to dissolve, and make an oil phase for later use; take PGE1 clathrate, monosialotetrahexosyl ganglioside (GM1), benzoic acid and add them to purified water, then add three Stir ethanolamine, Tween-80, glycerin, and propylene glycol evenly, and heat to 75°C to form a water phase; gradually add the water phase to the oil phase while stirring...
Embodiment 2
[0029] Element:
[0030] PGE1: 20mg, GM1: 10g;
[0031] HP-β-CD: 1.5g, petrolatum: 200g, polyacrylic acid: 50g, cetyl alcohol: 100g, triethanolamine: 5ml, Tween-80: 80g, glycerin: 50g, propylene glycol: 100g, benzoic acid: 0.5g, Absolute ethanol: 3ml, make up the balance with purified water.
[0032] Preparation method: Take PGE1 according to the prescription, add absolute ethanol to dissolve; take HP-β-CD and add purified water to dissolve, mix the two solutions, ultrasonically treat for 10 minutes, and freeze-dry. The obtained PGE1 inclusion compound is placed in a desiccator for later use; Polyacrylic acid, cetyl alcohol, heated to 75 ℃ to dissolve, and make an oil phase for later use; take PGE1 clathrate, monosialotetrahexosyl ganglioside (GM1), benzoic acid and add them to purified water, then add three Stir ethanolamine, Tween-80, glycerin, and propylene glycol evenly, and heat to 75°C to form a water phase; gradually add the water phase to the oil phase while stirring...
Embodiment 3
[0034] Element:
[0035] PGE1: 15mg, GM1: 20g;
[0036] HP-β-CD: 1.0g, petrolatum: 150g, polyacrylic acid: 50g, cetyl alcohol: 150g, triethanolamine: 5ml, Tween-80: 80g, glycerin: 75g, propylene glycol: 75g, benzoic acid: 0.8g, Absolute ethanol: 2ml, make up the balance with purified water.
[0037] Preparation method: Take PGE1 according to the prescription, add absolute ethanol to dissolve; take HP-β-CD and add purified water to dissolve, mix the two solutions, ultrasonically treat for 10 minutes, and freeze-dry. The obtained PGE1 inclusion compound is placed in a desiccator for later use; Polyacrylic acid, cetyl alcohol, heated to 75 ℃ to dissolve, and make an oil phase for later use; take PGE1 clathrate, monosialotetrahexosyl ganglioside (GM1), benzoic acid and add them to purified water, then add three Stir ethanolamine, Tween-80, glycerin, and propylene glycol evenly, and heat to 75°C to form a water phase; gradually add the water phase to the oil phase while stirring,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com