Preparation method of flucloxacillin sodium crystal form III
A technology of flucloxacillin sodium and crystal form, applied in the direction of organic chemistry, etc., can solve problems such as unstable solubility properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
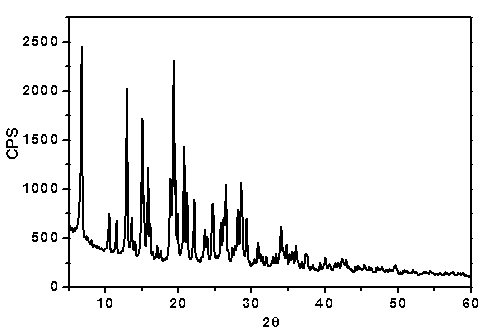
[0057] 1. Preparation of flucloxacillin sodium crystal form I:
[0058] Add 1.0 g of flucloxacillin sodium bulk drug and 17 mL of isopropanol into a 50 mL three-necked flask, stir with magnetic force at a stirring speed of 20 r / min. After heating at 50-55°C for 0.5h, filter while it is hot, collect the filtrate, cool and crystallize at room temperature, collect the crystals after precipitation and dry at a constant temperature of 50°C, dry for 12 hours and store in a desiccator sealed at room temperature.
[0059] 2. Confirmation of flucloxacillin sodium crystal form I:
[0060] 1. UV spectrophotometry:
[0061] Use phosphate buffer solution with pH=6.8 to configure 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form I solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0062] Operating conditions:
[0063] Detector: UV-2450 ultraviolet spectrophotometer
[0064] As a result, the crystalline form I of flucloxacil...
Embodiment 2
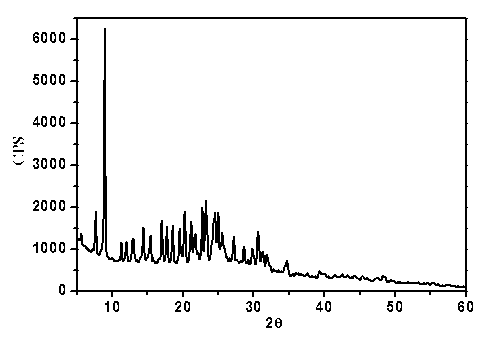
[0084] 1. Preparation of flucloxacillin sodium crystal form II:
[0085] Add 2.0 g of flucloxacillin sodium bulk drug and 20 mL of ethanol into a 50 mL three-necked flask, and stir magnetically at a stirring speed of 20 r / min. After heating under boiling conditions for 0.5h, filter while it is hot, slowly cool and crystallize, collect the crystals after precipitation and dry at a constant temperature of 50°C, dry for 12 hours and store in a desiccator sealed at room temperature.
[0086] 2. Confirmation of flucloxacillin sodium crystal form II:
[0087] 1. UV spectrophotometry:
[0088] Use phosphate buffer solution with pH=6.8 to configure 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form II solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0089] Operating conditions:
[0090] Detector: UV-2450 ultraviolet spectrophotometer
[0091]As a result, the crystalline form II of flucloxacillin sodium obtained a...
Embodiment 3
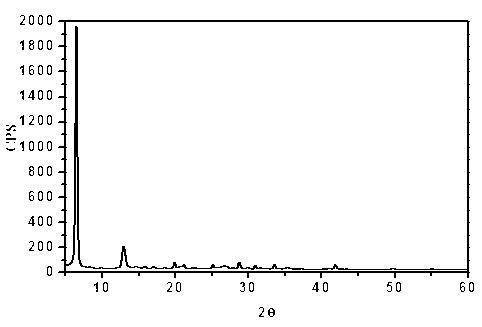
[0111] 1. Preparation of flucloxacillin sodium crystal form III:
[0112] Add 1.0 g of flucloxacillin sodium bulk drug and 13 mL of acetone into a 50 mL three-necked flask, stir with magnetic force at a stirring speed of 20 r / min. After heating under boiling conditions for 0.5h, filter while it is hot, cool and crystallize at a lower temperature (5-15°C), collect the crystals after precipitation and dry at a constant temperature of 50°C, dry for 12 hours and seal them in a desiccator at room temperature.
[0113] 2. Confirmation of flucloxacillin sodium crystal form III:
[0114] 1. UV spectrophotometry:
[0115] Use phosphate buffer solution with pH=6.8 to configure 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form III solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0116] Operating conditions:
[0117] Detector: UV-2450 ultraviolet spectrophotometer
[0118] As a result, the crystal form III of flucloxa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com