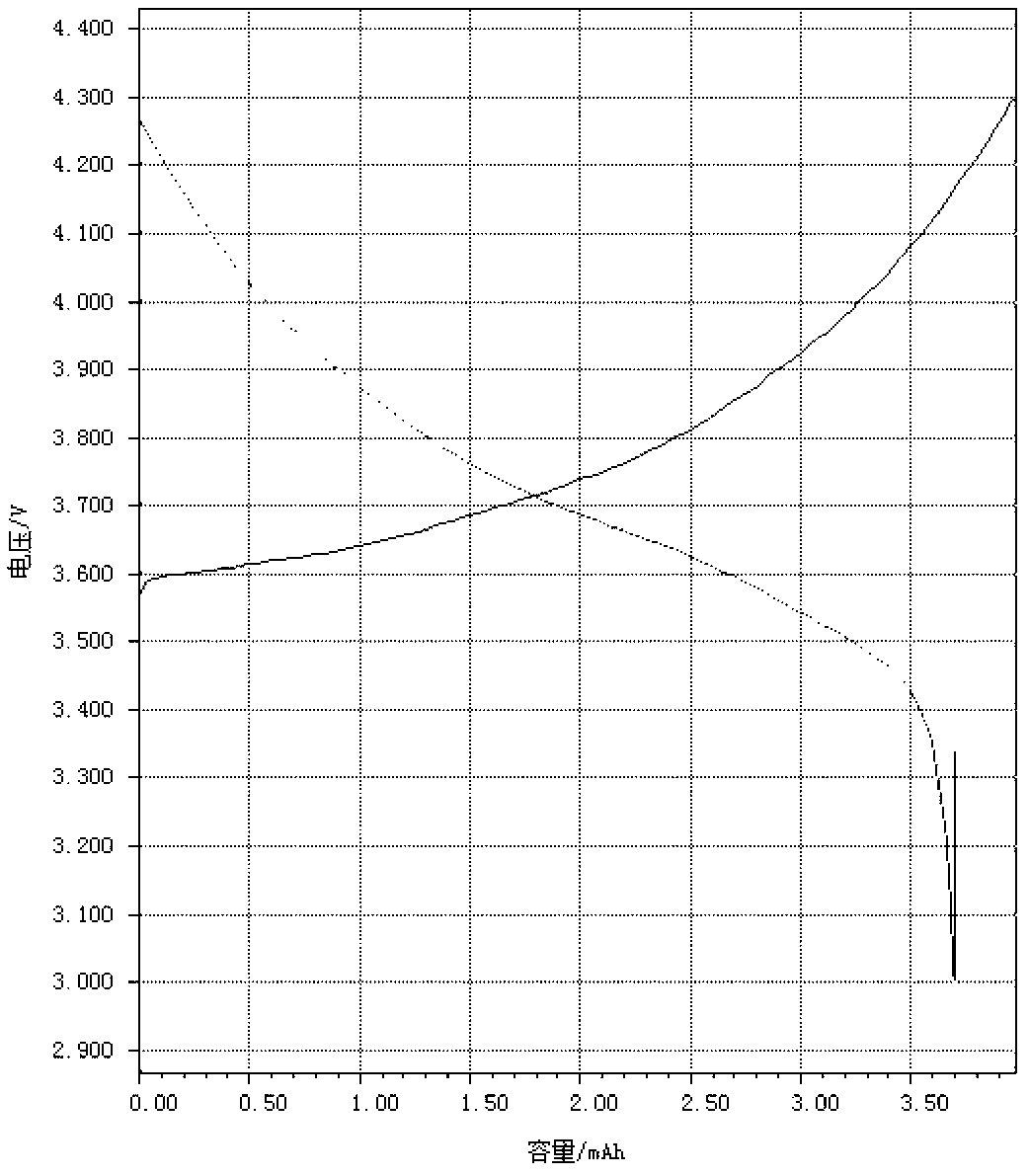
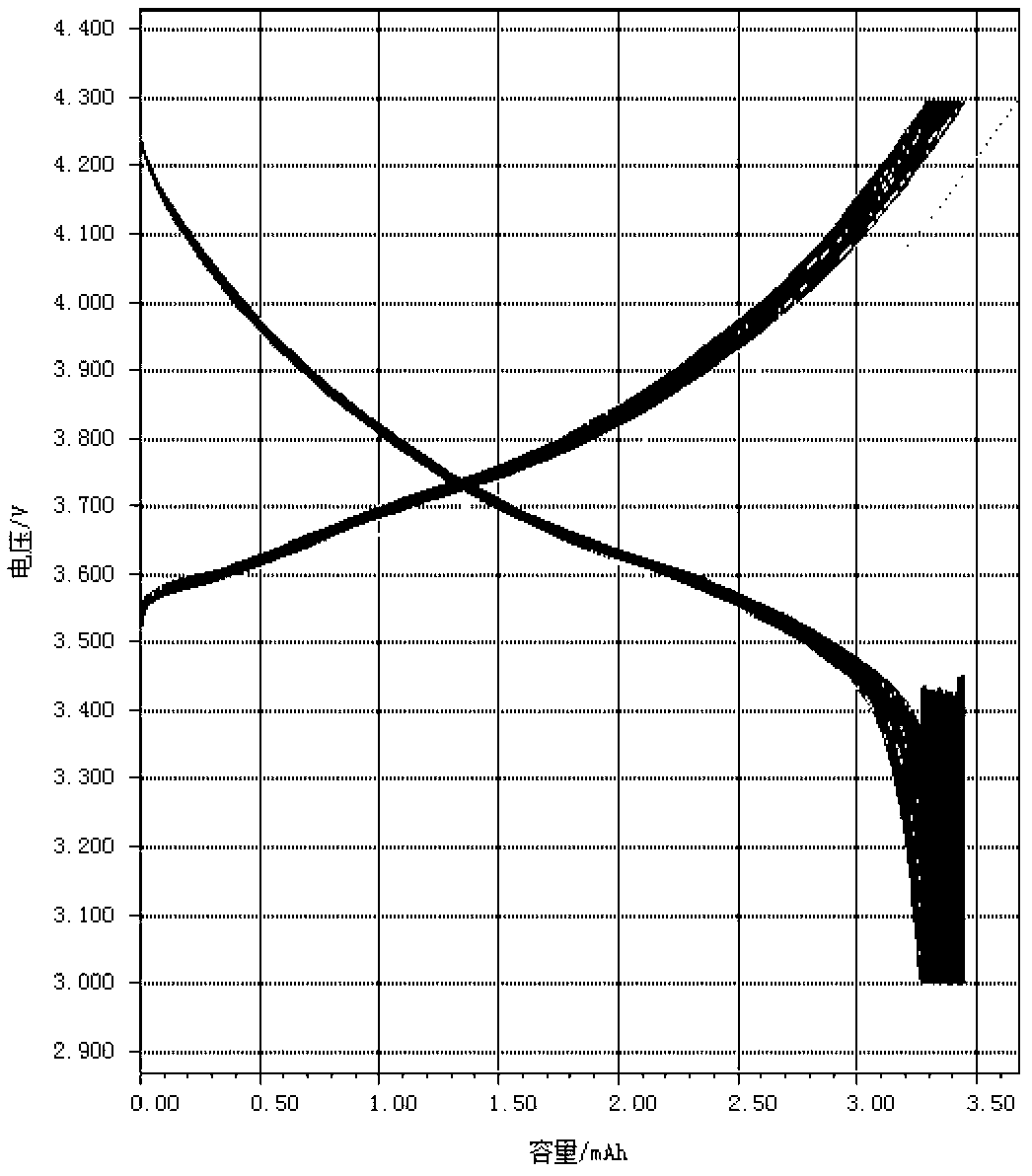
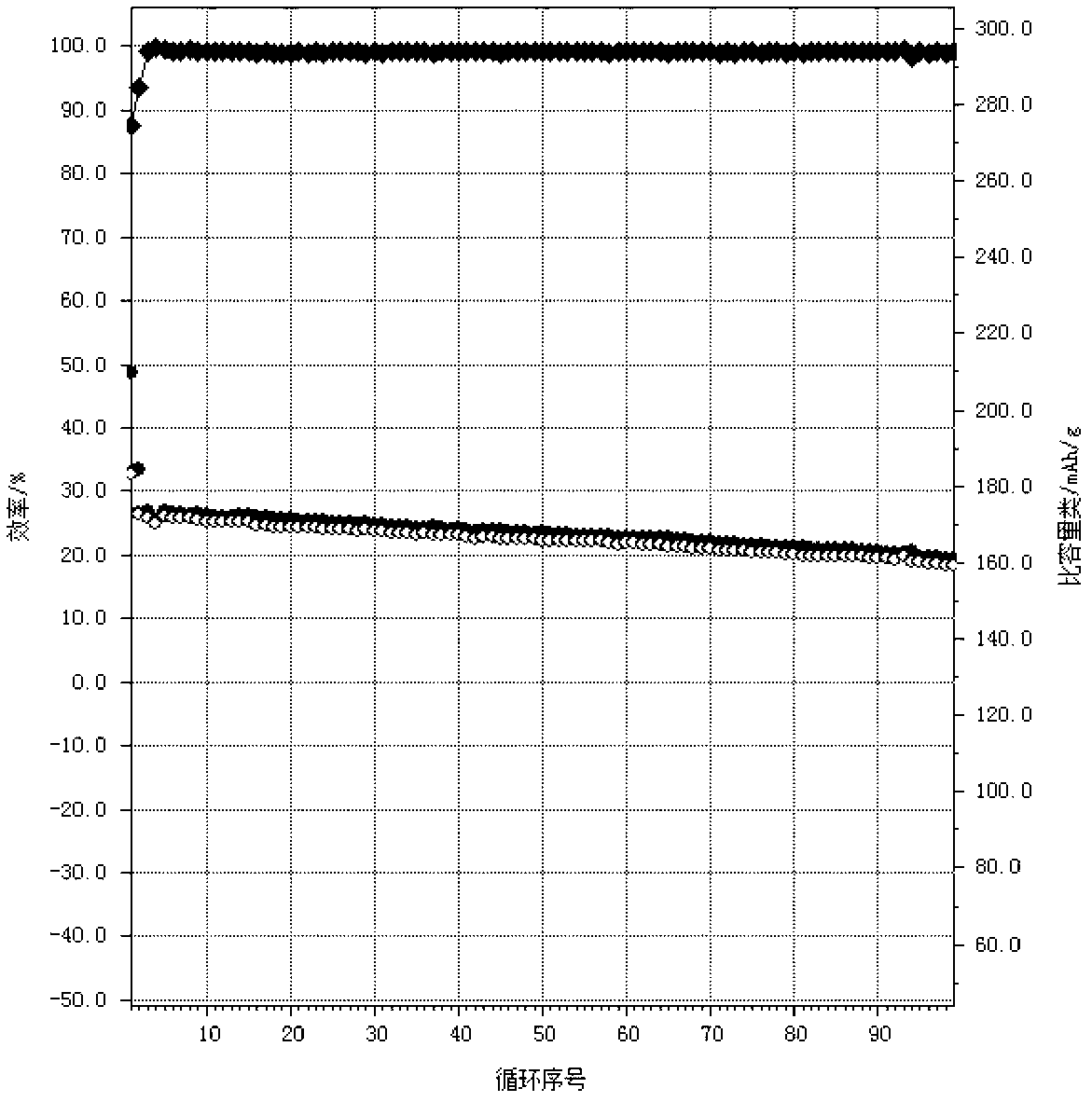
Coated nickel lithium cobalt oxide positive material with high capacity, low residual alkali and low pH value, and preparation method thereof
A positive electrode material, lithium nickel cobalt oxide technology, applied in the direction of battery electrodes, electrical components, circuits, etc., can solve the problems of material internal structure collapse, high residual alkali on the product surface, cycle performance decline, etc., to achieve low cost and easy battery technology , the effect of high tap density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 65g of nickel hydroxide into a 1L reaction kettle, add 100g of prepared 10wt% ammonia solution into the reaction kettle, stir for half an hour and mix well. Use a peristaltic pump to slowly add 200ml of the configured 1.5mol / L cobalt sulfate solution into the reactor. At the same time, use a peristaltic pump to add 0.5mol / L sodium hydroxide solution at a uniform speed, and keep the solution during the feeding. The pH value was maintained at 11, until the cobalt sulfate solution was added and the lye feeding was stopped, and the stirring was continued for 3 hours. After aging for 12 hours, the temperature was maintained at 65°C during the reaction, and the stirring speed was 400r / min to obtain [Co(OH) 2 ] 0.3 ·[Ni(OH) 2 ] 0.7 The precursor was washed with deionized water until the pH was 8, and dried in a drying oven at 100 °C. Lithium hydroxide is added according to the total molar ratio of lithium to metal nickel-cobalt atoms at 1.00, mixed evenly, pretreated a...
Embodiment 2
[0025] Add 65g of nickel hydroxide into a 1L reactor, and add 100g of prepared 10wt% ammonia solution into the reactor. Mix well after stirring for half an hour. Use a peristaltic pump to slowly add 100ml of the prepared 3mol / L cobalt sulfate solution into the reactor at a certain speed. At the same time, use a peristaltic pump to add 0.5mol / L sodium hydroxide solution at a uniform speed. During this period, keep the pH value of the solution at 11, stop feeding until the cobalt sulfate solution is added, and continue to stir for 5 hours. After aging for 10 hours, the temperature was maintained at 65°C during the reaction, and the stirring speed was 400r / min to obtain [Co(OH) 2 ] 0.3 ·[Ni(OH) 2 ] 0.7 The precursor was washed with deionized water until the pH was 8, and dried in a drying oven at 100 °C. Lithium nitrate is added according to the total molar ratio of lithium to metal nickel and cobalt as 1.00, mixed evenly, pretreated at 520°C for 5 hours, cooled and ground, ...
Embodiment 3
[0027] Add 70g of nickel hydroxide into a 1L reactor, and add 100g of a prepared 30wt% ammonia solution into the reactor. Mix well after stirring for half an hour. Use a peristaltic pump to slowly add 250ml of 1mol / L cobalt sulfate solution into the reaction kettle at a certain speed. At the same time, use a peristaltic pump to add 1mol / L sodium hydroxide solution at a uniform speed. During the feeding Keep the pH value of the solution at 11, stop feeding until the cobalt sulfate solution is added, and continue to stir for 3 hours. After aging for 18 hours, the temperature was maintained at 55°C during the reaction, and the stirring speed was 400r / min to obtain [Co(OH) 2 ] 0.25 ·[Ni(OH) 2 ] 0.75 The precursor was washed with deionized water until the pH was 8, and dried in a drying oven at 100 °C. Lithium nitrate is added according to the total molar ratio of lithium to metal nickel and cobalt as 1.00, mixed evenly, pretreated at 520°C for 5h, cooled and ground, and calci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com