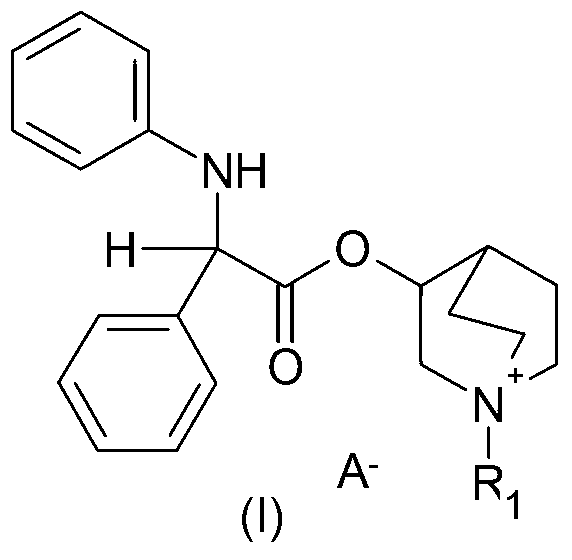
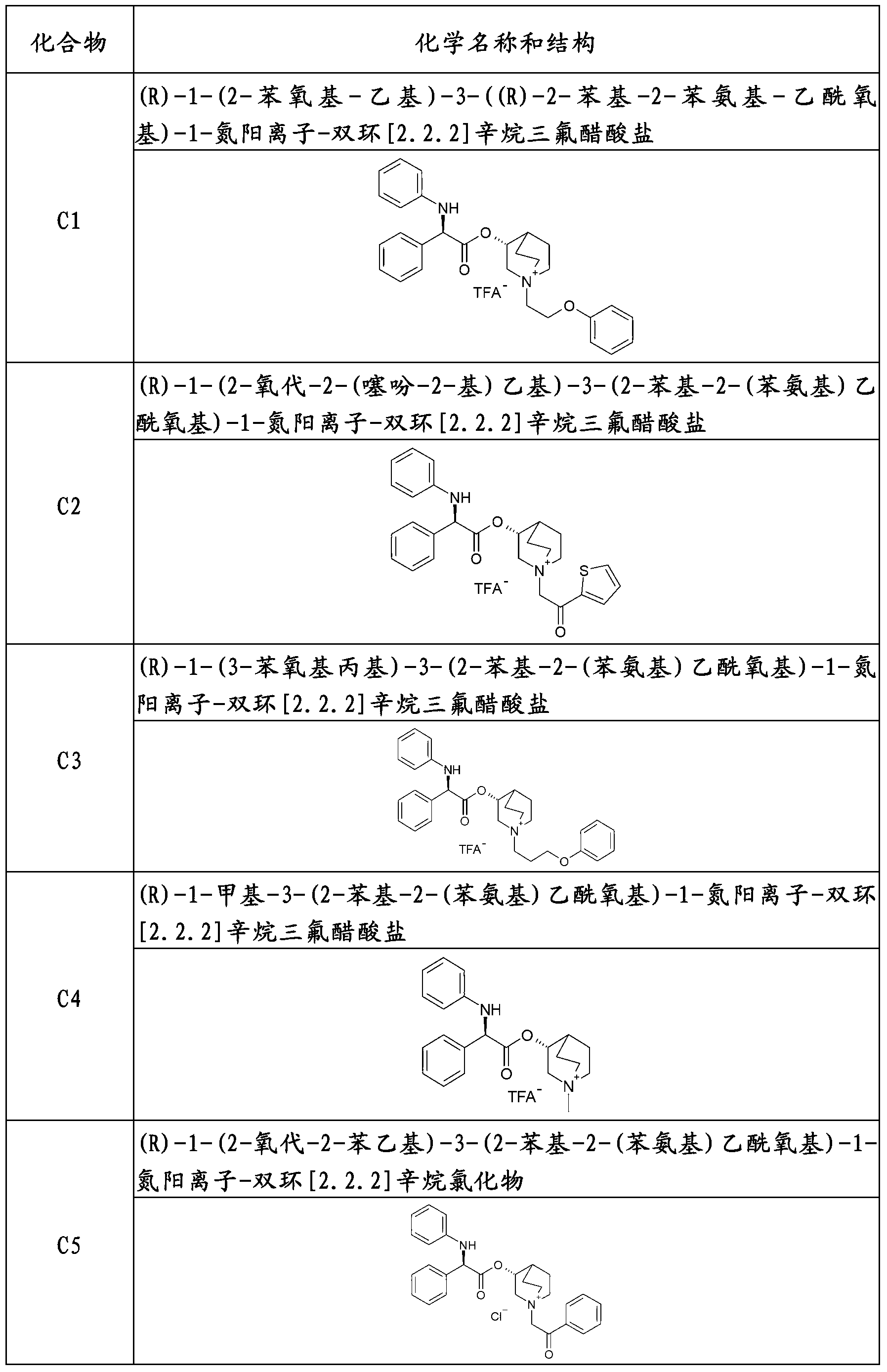
Liquid propellant-free formulation comprising an antimuscarinic drug
A propellant and preparation technology, which is applied in the field of preparation of the preparation, can solve problems such as the chemical stability of amino ester derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
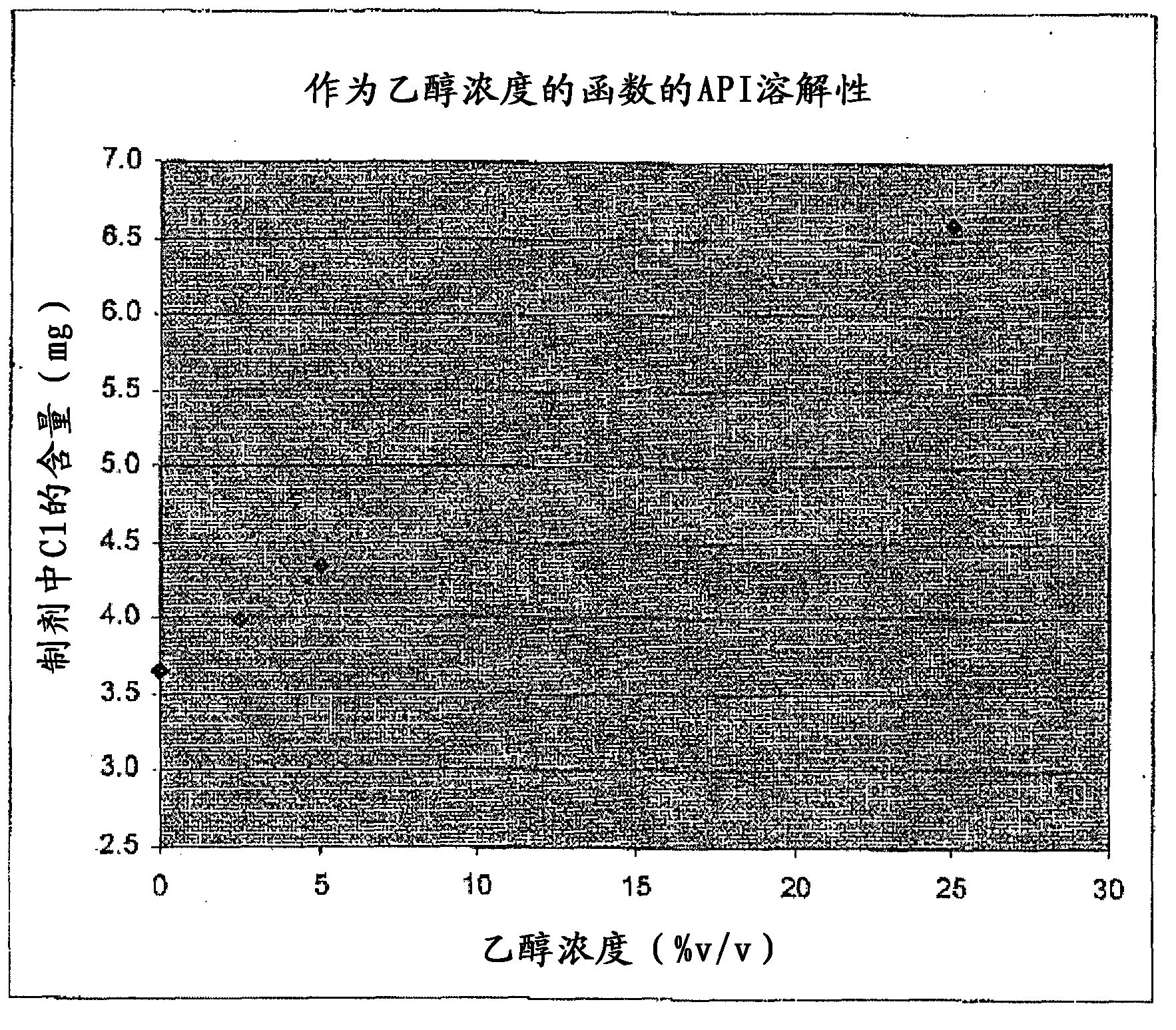
[0114] Example 1 - Determination of the Solubility of Active Ingredient C1 in Water-Ethanol Mixtures
[0115] The solubility of C1 in water / ethanol mixtures was determined as follows. Prepare vials containing excess C1 with 0%, 2.5%, 5% and 25% ethanol in water.
[0116] After equilibration, the samples were filtered through a 0.2 μm filter.
[0117] Record the results in the attached picture of picture , from which the C1 solubility can be extrapolated.
Embodiment 2
[0118] Example 2 - Propellant-free liquid formulation comprising C1 as active ingredient and ethanol solvent agent
[0119] A 10 mM citrate buffer containing 5.0% (v / v) ethanol was prepared and the pH was adjusted to 4.5 using 1 N sodium hydroxide.
[0120] 7 mg of C1 was weighed into a vial and 2 ml of 10 mM citrate buffer, pH 4.0 containing 5.0% ethanol was added. The solution was stirred, vortexed for 30 s every 5 min for 45 min, and then filtered using a 0.2 μm filter. Osmolarity was shown to be approximately 290 mOsm / kg.
Embodiment 3
[0121] Example 3 - Inhibitors containing C1 as active ingredient and propylene glycol as co-solvent Injection liquid preparation
[0122] Three 10 mM citrate buffers containing 1.85% (v / v) propylene glycol (PG) were prepared and the pH was adjusted to 4.0, 5.0 and 6.0 using 1 N sodium hydroxide.
[0123] 2, 3 and 4 mg of Cl were weighed into separate vials and 1 ml of 10 mM citrate buffer, pH 4.0 containing 1.85% propylene glycol was added. Each solution was stirred, vortexed for 30 s every 5 min for 45 min, and then filtered using a 0.2 μm filter. pH and osmolarity were then tested.
[0124] Similar formulations containing C1 as the active ingredient were prepared but using starting solutions in which the pH was 5.0 and 6.0, respectively. All prepared formulations are reported in Table 1.
[0125] Table 1 - Composition of the formulations
[0126]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com