Preparation method for methyl nitrite
A technology of methyl nitrite and nitric acid, which is applied in the field of preparation of methyl nitrite, can solve the problems of no nitrite and can not completely eliminate the generation and discharge of nitric acid, so as to improve the yield, reduce the heat supply of the tower kettle, Conducive to the effect of environmental protection requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
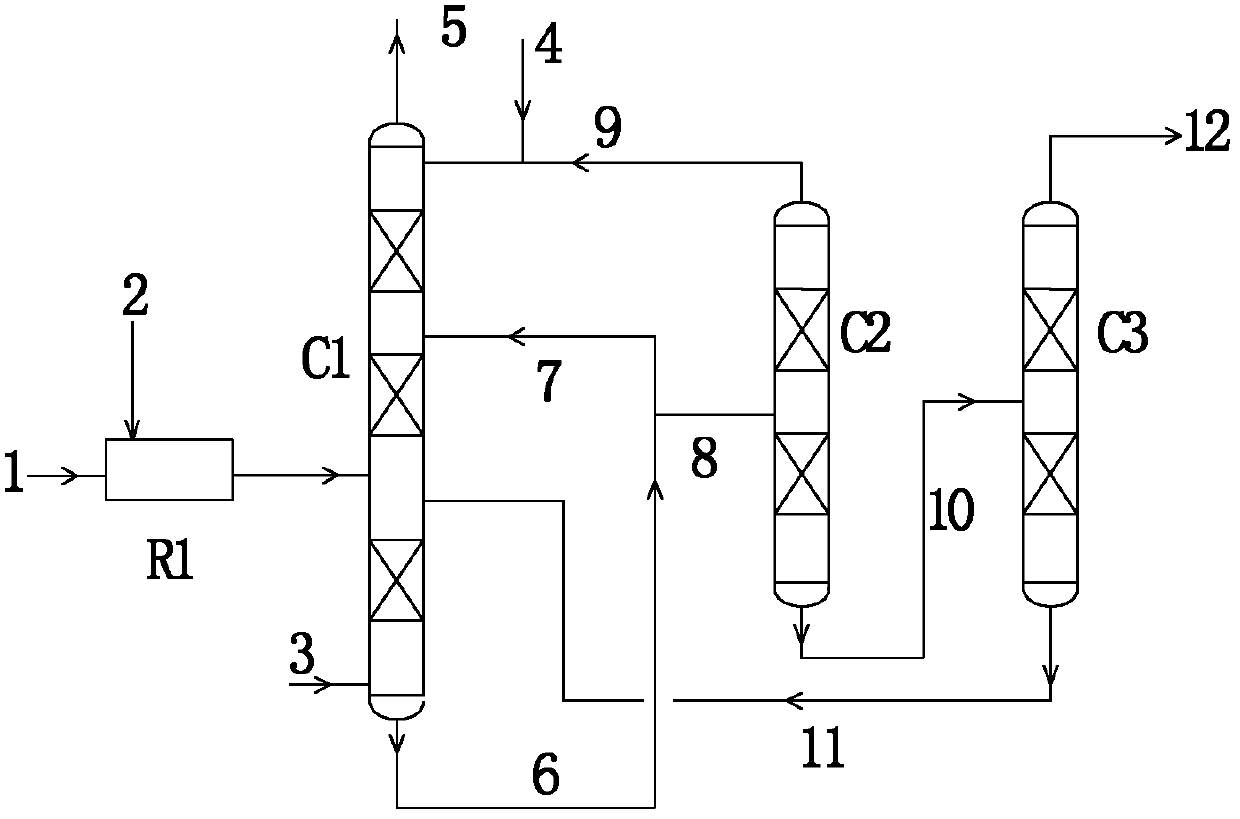
[0041] A flow of 100kmol / hr containing NO gas (wherein NO content 14vol%, the rest is N 2 ) and O 2 After the reaction, 6vol% N 2 o 3 , the mixed gas containing 2vol% NO and nitrogen (the rest) enters the reaction section of the esterification tower, and is in countercurrent contact with methanol, water and nitric acid flowing down from top to bottom, and another stream of NO-containing gas with a flow rate of 20kmol / hr (the content of NO is 14vol%, and the rest is N 2 ) flows from the bottom of the stripping section, that is, the bottom of the esterification tower, to the reaction section from bottom to top. The pressure of the esterification tower kettle is 400KPa, and the temperature is 79°C. The gas discharged from the top of the esterification tower is a mixture of MN, NO and nitrogen.
[0042] A large amount of methanol-containing nitric acid wastewater produced in the esterification tower and tower kettle, part of which is directly refluxed to the bottom reaction s...
Embodiment 2
[0045] A flow of 100kmol / hr containing NO gas (wherein NO content 14vol%, MN content is 4vol%, the rest is N 2 ) and O 2 After the reaction, 6vol% N 2 o 3 , containing 2vol% NO, containing 4vol% MN, and the mixed gas of nitrogen (the rest), enters the reaction section of the esterification tower, and contacts with the methanol, water and nitric acid flowing down from top to bottom, and the other flow is 20kmol / hr of NO-containing gas (the content of NO is 14vol%, the content of MN is 4vol%, and the rest is N 2 ) flows from the bottom of the stripping section, that is, the bottom of the esterification tower, to the reaction section from bottom to top. The pressure of the esterification tower is 300KPa, and the temperature is 73°C. The gas discharged from the top of the esterification tower is a mixture of MN, NO and nitrogen.
[0046] A large amount of methanol-containing nitric acid wastewater produced in the esterification tower reactor, part of which is directly reflux...
Embodiment 4
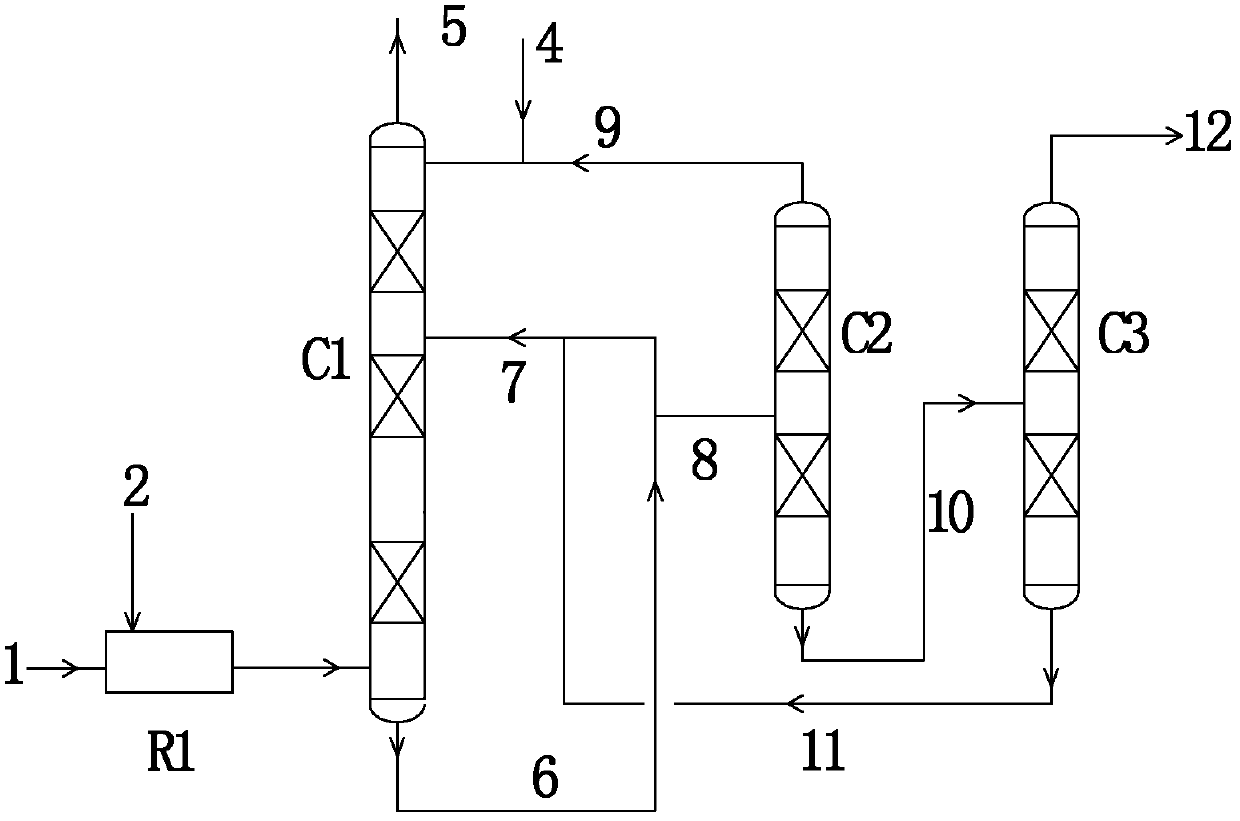
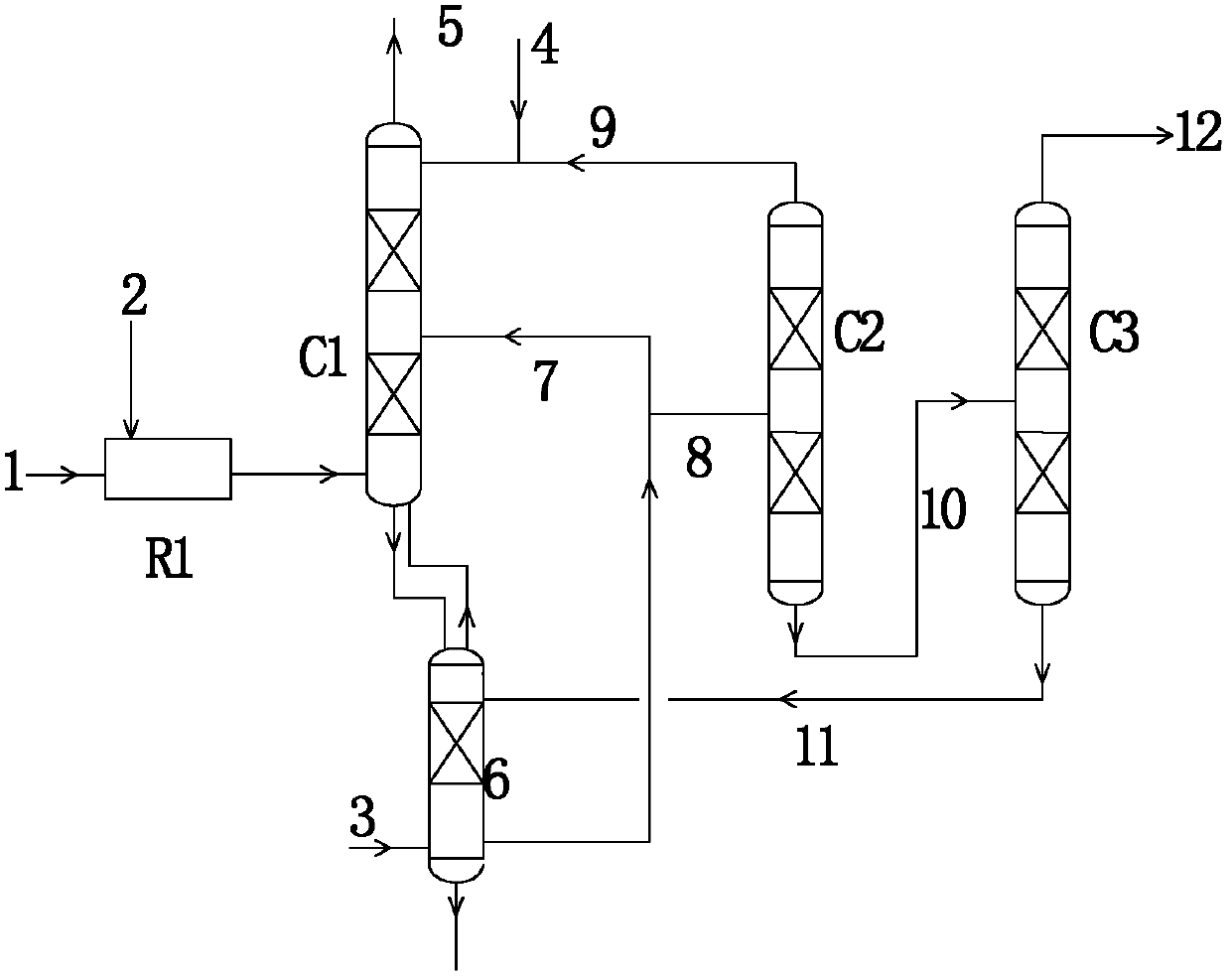
[0057] A preparation method of methyl nitrite, specifically comprising the following steps:
[0058] a) Mix the first stream of NO-containing gas with O 2 Directly input into the reaction section of the esterification tower, methanol is input from the top of the esterification tower, and the second NO-containing gas is input from the bottom of the esterification tower;
[0059] b) The gas in the reaction section of the esterification tower flows through the reaction section from bottom to top, reacts with the methanol flowing down from top to bottom for esterification reaction, and generates methyl nitrite, water and by-product nitric acid;
[0060] c) The liquid phase mixture of nitric acid, methanol and water flowing down from the reaction section of the esterification tower and the methyl nitrite dissolved in the liquid phase mixture enter the gas stripping section of the esterification tower, and carry out countercurrent with the second stream of NO-containing gas Contact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com