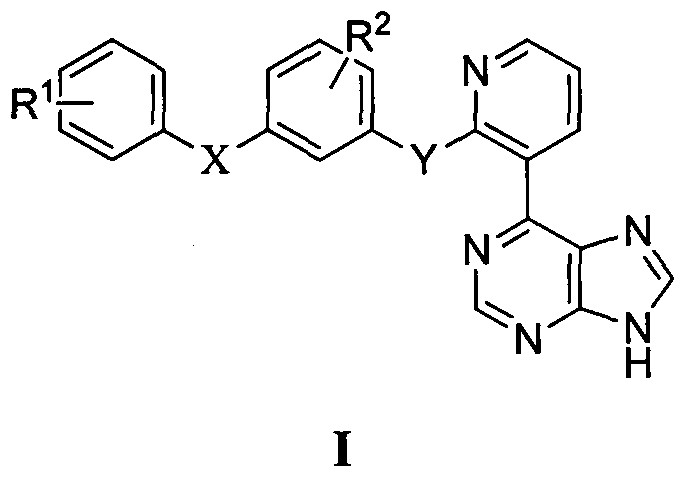
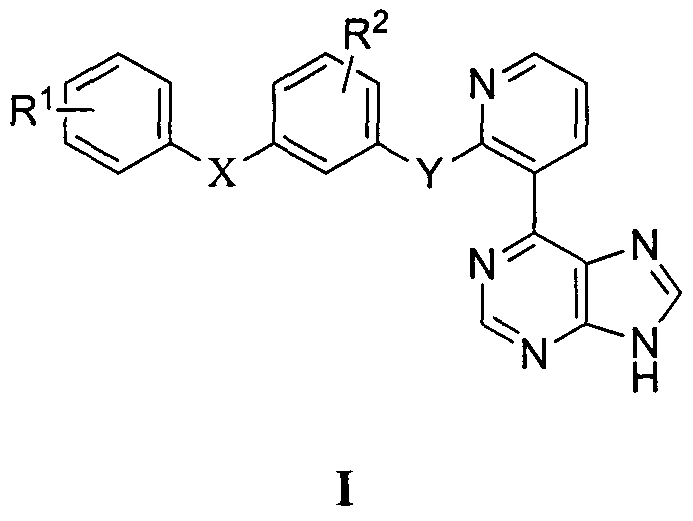
Aramide Raf kinase inhibitor based on purine structure and preparation method and application thereof
A purine and benzamide technology, applied in organic chemistry, antineoplastic drugs, extracellular fluid diseases, etc., can solve the problems of easy transfer and diffusion, low cure rate, and easy recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
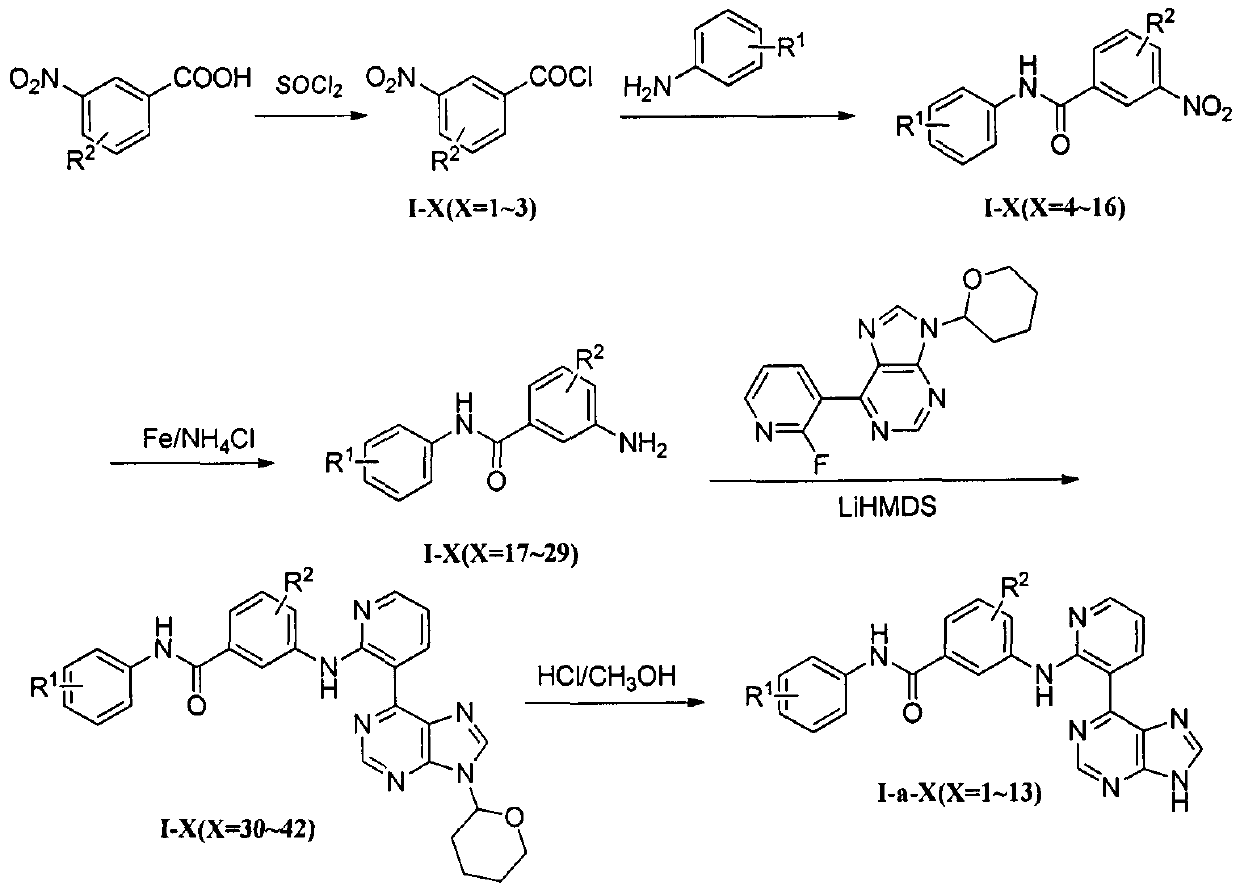
Embodiment 1
[0108] 6-Chloro-9-(tetrahydro-2H-pyran-2-yl)-9H-purine (P-1)
[0109] Add 2.0g (13mmol) of 6-chloro-9H-purine, 3.3g (39mmol) of 3,4-dihydro-2H-pyran, 50mg (0.3mmol) of p-toluenesulfonic acid and no Add 30 mL of ethyl acetate in water, stir, and heat to reflux for 3 hours. After cooling, excess ethyl acetate was distilled off under reduced pressure to obtain 3.0 g of light yellow oil (P-1), with a yield of 96.7% and a literature yield of 99.3%. The product does not need to be purified and is directly used for the next reaction.
Embodiment 2
[0111] 6-(2-fluoro-3-pyridyl)-9-(tetrahydro-2H-pyran-2-yl)-9H-purine (P-2)
[0112] Add P-13.0g (13mmol), 2-fluoro-3-pyridine boronic acid 2.7g (19.5mmol), NaCO 3 5.5g (51mmol), PdCl 2 (dppf) 180mg (0.24mmol), water 5mL and 1,4-dioxane 35mL, under stirring, N 2 Protection, reflux reaction for 14hr. After cooling, dissolve the reaction solution in 50mL water and 50mL ethyl acetate, let stand, separate the layers, collect the organic layer, extract the water layer with ethyl acetate three times (50mL×3), combine the organic layers and wash with 50mL saturated saline Wash, dry over anhydrous sodium sulfate, filter with suction, and concentrate to obtain the crude product. After silica gel column chromatography (developing solvent: ethyl acetate:petroleum ether=1:1), 2.0 g of light yellow solid (P-2) was obtained, yield 51.5%, mp: 125~127°C. MS(LR-ESI): 300.2[M+H] + . 1 HNMR (300MHz, CDCl 3 ): δ1.70~2.23 (6H, m, CH 2 ), 3.82 (1H, t, CH2 O, J=8.2Hz), 4.21 (1H, d, CH 2 O, J...
Embodiment 3
[0114] 4-Methyl-3-nitrobenzoyl chloride (I-1)
[0115] Add 725 mg (4 mmol) of 4-methyl-3-nitrobenzoic acid, 15 mL of thionyl chloride and 2 drops of N, N-dimethylformamide (DMF) into a 100 mL eggplant-shaped flask, and react under reflux for 5 hr with stirring. After cooling, the thionyl chloride was distilled off under reduced pressure to obtain 790 mg of light yellow oil (I-1), with a yield of 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com