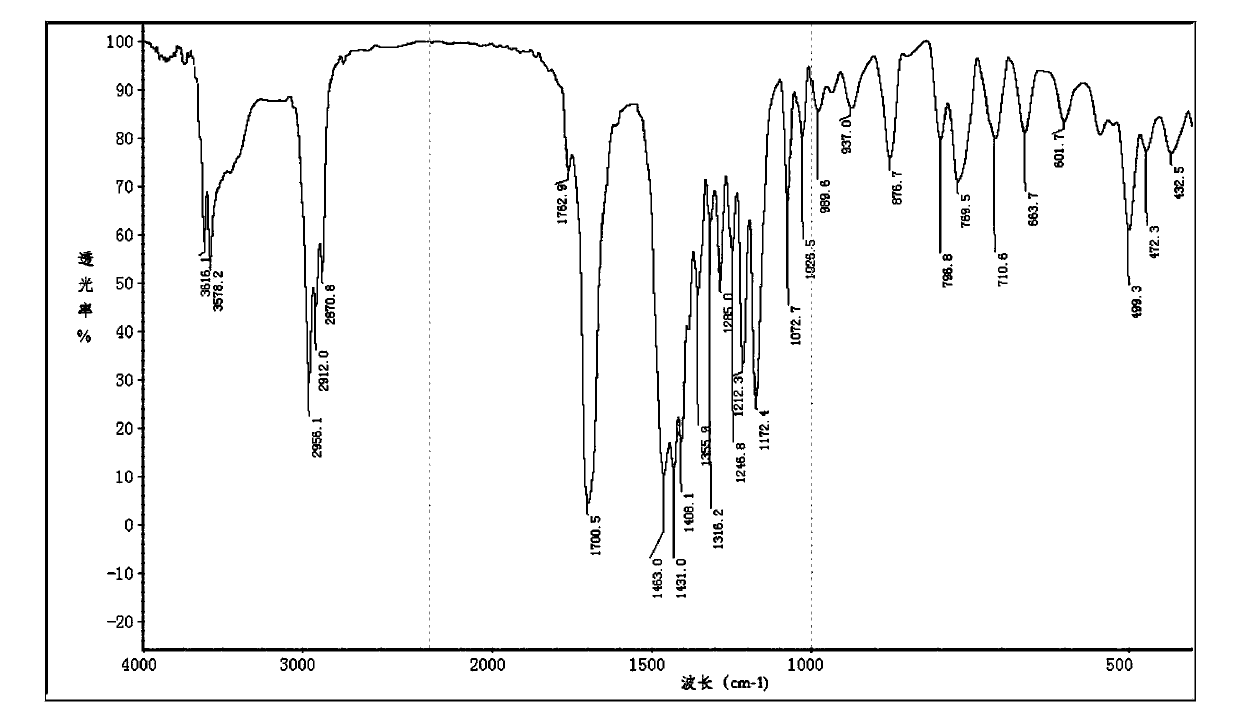
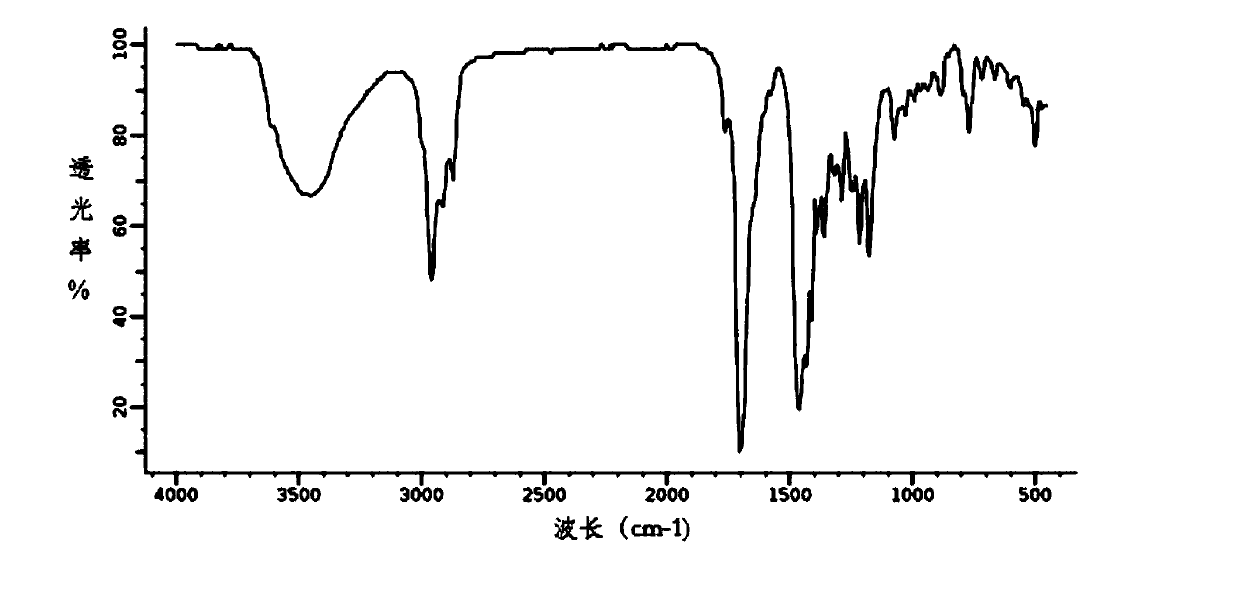
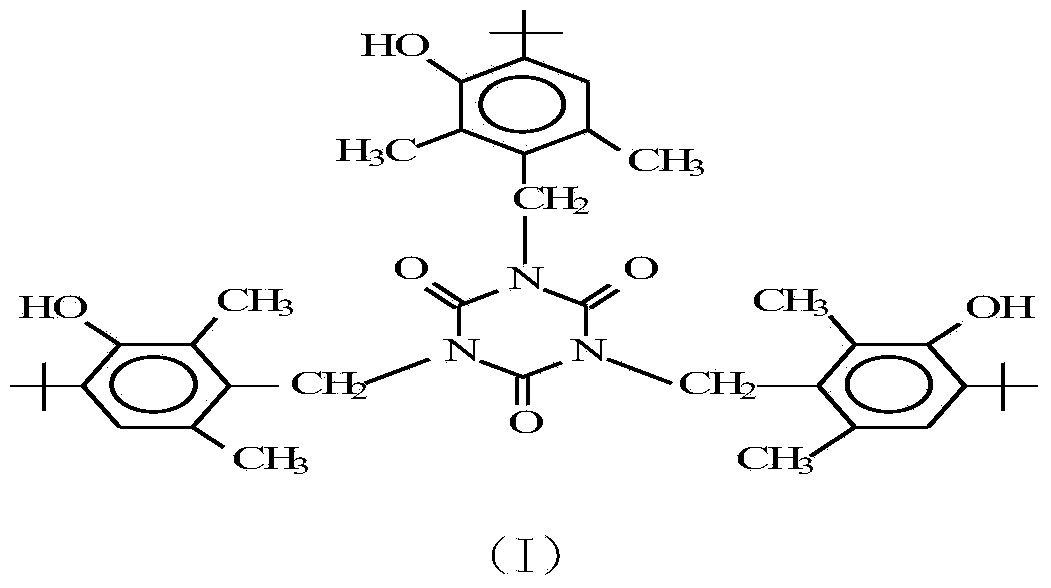
Method for preparing 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate
A technology of dimethylbenzyl and isocyanurate, applied in the direction of organic chemistry, can solve the problems of increased production cost, uneconomical, high processing cost, etc., and achieve the goal of simplifying the production operation process, reducing costs, and reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 500ml four-neck flask equipped with a stirrer, reflux condenser, and thermometer, add 19.8g (0.1mol) of trisodium cyanurate, 4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl Chlorine 71g (0.31mol), N,N-dimethylformamide 200ml and phase transfer catalyst 1g, nitrogen protection was continuously passed through, stirring was raised to 120°C, and the reaction was kept for 14h. Then cool down to 50-60°C and filter, the filtrate is distilled under reduced pressure of 0.005-0.015MPa to remove N,N-dimethylformamide, add 200ml of toluene and 100ml of water to the residue, stir and heat up until the material is completely dissolved, static Separate the water phase, distill the remaining water and about 160ml of toluene from the organic phase, slowly add 150ml of methanol to the residue, dissolve the material under stirring and reflux, add 5g of activated carbon for decolorization, heat filter, cool down and crystallize, filter, rinse with methanol, and dry 54.6 g of 1,3,5-tris(4-te...
Embodiment 2
[0028] In the same four-neck flask as in Example 1, add 19.8 g (0.1 mol) of trisodium cyanurate, 71 g (0.31 mol) of 4-tert-butyl-3-hydroxyl-2,6-dimethylbenzyl chloride , 200ml of N,N-dimethylacetamide and 1g of phase transfer catalyst, continuously fed with nitrogen protection, stirring and raising the temperature to 120°C, and keeping the reaction for 14h. Then cool down to 50-60°C and filter, the filtrate is distilled under reduced pressure of 0.005-0.015MPa to remove N,N-dimethylacetamide, add 200ml xylene and 100ml water to the residue, stir and heat up until the material is completely dissolved, Stand still to separate the water phase, distill the remaining water and about 150ml of xylene from the organic phase, slowly add 150ml of methanol to the residue, stir and reflux the material to dissolve, add 5g of activated carbon for decolorization, heat filter, cool down and crystallize, filter, rinse with methanol, 52.5 g of dry 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylb...
Embodiment 3
[0030] In the same four-neck flask as in Example 1, 19.8 g (0.1 mol) of trisodium cyanurate, 75.5 g (0.33 mol) of 4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl chloride were added ), N, N-dimethylformamide 200ml and a phase transfer catalyst 2g, continue to feed nitrogen protection, stir and raise the temperature to 100°C, and keep the reaction for 20h. Then cool down to 50-60°C and filter, the filtrate is distilled under reduced pressure of 0.005-0.015MPa to remove N,N-dimethylformamide, add 200ml of toluene and 100ml of water to the residue, stir and heat up until the material is completely dissolved, static Separate the water phase, distill the remaining water and about 160ml of toluene from the organic phase, slowly add 150ml of methanol to the residue, dissolve the material under stirring and reflux, add 5g of activated carbon for decolorization, cool down and crystallize, filter, rinse with methanol to obtain dry 1, 50.8 g of 3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com