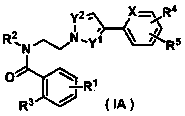
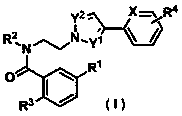
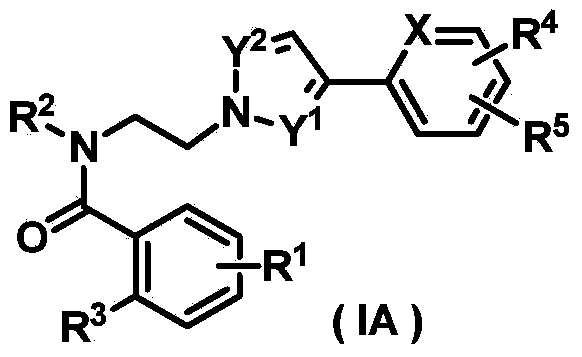
Pyrazole derivative
A technology of pyrazole derivatives and triazolyl, which is applied in the field of pyrazole derivatives and can solve the problems of undisclosed OX receptor antagonism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0216] Hereinafter, the present invention will be described in more detail with reference to Reference Examples, Examples, and Test Examples. However, these examples are not intended to limit the invention. Furthermore, modifications or variations may be made to the present invention without departing from the scope of the present invention.
[0217] In the following Reference Examples and Examples, the microwave reactor used was an Initiator from Biotage Japan Ltd.
[0218] In the following Reference Examples and Examples, SNAPCartridgeKP-Sil from Biotage Japan Ltd. was used as "KP-Sil" for purification using column chromatography; SNAPCartridgeHP-Sil from Biotage Japan Ltd. was used as "HP-Sil" and SNAPCartridge KP-NH from Biotage Japan Ltd. was used as "KP-NH".
[0219] In the following Reference Examples and Examples, purification by preparative high performance liquid chromatography (HPLC) was performed under the conditions shown below. However, in the case of a compou...
Embodiment 2
[0249] Referring to Example 25-fluoro-2-(1H-pyrazol-5-yl)pyridine hydrochloride
[0250] [Formula 13]
[0251]
[0252] 4mol / L HCl-EtOAc solution (5 mL) was added to a solution of the compound (0.84 g, 3.4 mmol) obtained in Reference Example 1 in MeOH (10 mL), and the mixture was stirred at room temperature for 16 hours and heated at 60° C. Stir for 3 hours. After standing to cool at room temperature, the solvent in the reaction mixture was distilled off under reduced pressure. The obtained residue was dissolved in Et 2 O for 1 hour. Then, the deposited solid was collected by filtration and dried by heating under reduced pressure to obtain the title compound (0.62 g) (colorless powder).
[0253] MS (ESI positive ion) m / z: 164[M+H]+, 186[M+Na]+
[0254] Referring to Example 3 {2-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]ethyl}carbamate tert-butyl ester
[0255] [Formula 14]
[0256]
[0257] N-(2-bromoethyl) tert-butyl carbamate (1.9g, 8.5mmol) and Cs 2 CO 3 (6.4...
Embodiment 4
[0259] Referring to Example 42-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]ethylamine dihydrochloride
[0260] [Formula 15]
[0261]
[0262] 4mol / L HCl-EtOAc solution (5mL) was added to the compound obtained in reference example 3 (1.3g, 4.2mmol) in CHCl 3 (20 mL), and the mixture was stirred at room temperature for 24 hours. The solvent in the reaction mixture was distilled off under reduced pressure, and the obtained residue was dissolved in Et 2 O for 1 hour. Then, the deposited solid was collected by filtration and dried by heating under reduced pressure to obtain the title compound (0.86 g) (colorless powder).
[0263] MS (ESI positive ion) m / z:207[M+H]+
[0264] Referring to Example 55-fluoro-2-(1H-pyrazol-4-yl)pyridine
[0265] [Formula 16]
[0266]
[0267] 2mol / LNa 2 CO 3 Aqueous solution (8.5mL, 17.1mmol) and Pd(PPh 3 ) 4 (0.20g, 0.17mmol) was added to 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole-1-carboxylate A solution of tert-butyl e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com