Liranaftate ointment and preparation method thereof
A technology of liranaphtate and ointment, which is applied in the field of pharmaceutical preparations, can solve problems such as inability to ensure drug uniformity, non-compliant particle size, and affect the efficacy of preparations, and achieve the effects of easy quality control, uniform content, and fewer types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
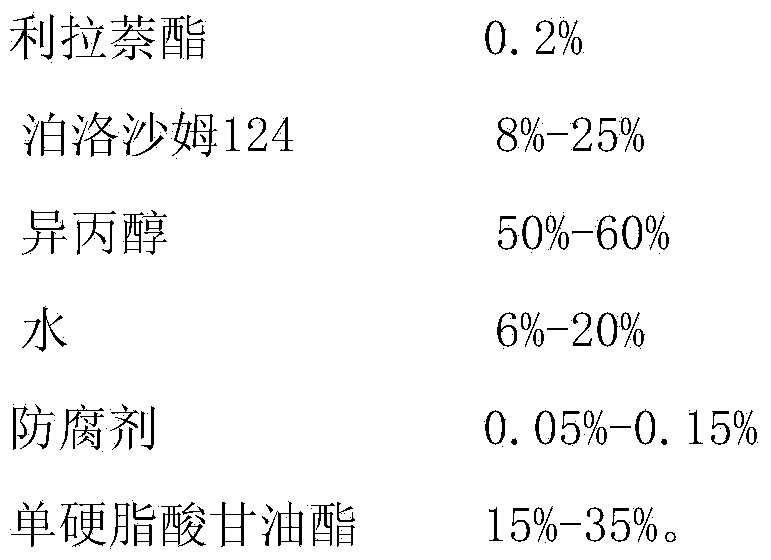
[0029] Embodiment 1 liranaphtate ointment prescription and preparation technology
[0030]
[0031] Preparation Process:
[0032] Add liranaphtate into poloxamer 124, stir to dissolve, and use it as the internal oil phase; mix isopropanol, water and benzyl alcohol evenly, and use it as the water phase; mix the internal oil phase and the water phase to obtain a uniform Drug-containing solution: heat and melt glycerol monostearate at 80°C as the outer oil phase, mix the outer oil phase with the drug-containing solution, and stir evenly to obtain the product.
Embodiment 2
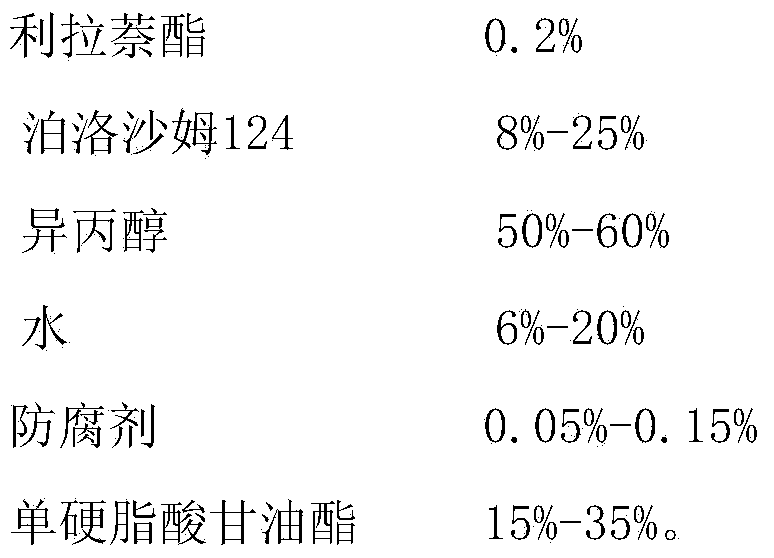
[0033] Embodiment 2 liranaphtate ointment prescription and preparation technology
[0034]
[0035] Preparation Process:
[0036] Add liranaphtate into poloxamer 124, stir to dissolve, and use it as the internal oil phase; mix isopropanol, water and sodium benzoate evenly, and use it as the water phase; mix the internal oil phase and the water phase to obtain a uniform Drug-containing solution: heat and melt glycerol monostearate at 75°C as the outer oil phase, mix the outer oil phase with the drug-containing solution, and stir evenly to obtain the product.
Embodiment 3
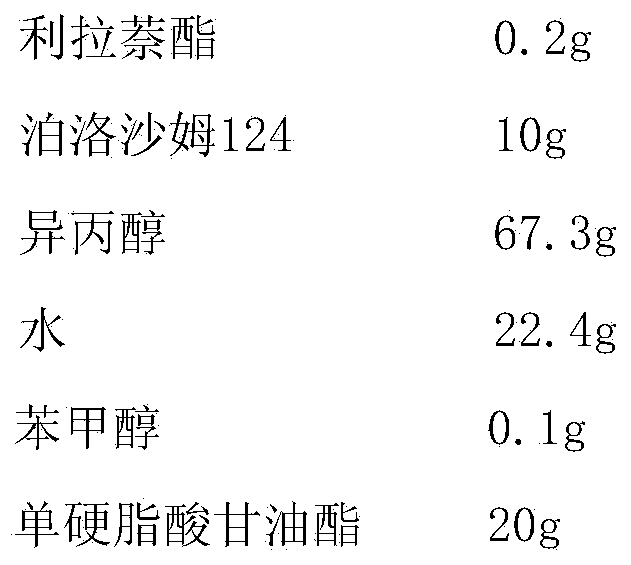
[0037] Embodiment 3 liranaphtate ointment prescription and preparation technology
[0038]
[0039] Preparation Process:
[0040] Add liranaphtate into poloxamer 124, stir to dissolve, and use it as the internal oil phase; mix isopropanol, water and sodium benzoate evenly, and use it as the water phase; mix the internal oil phase and the water phase to obtain a uniform Drug-containing solution: heat and melt glycerol monostearate at 75°C as the outer oil phase, mix the outer oil phase with the drug-containing solution, and stir evenly to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com