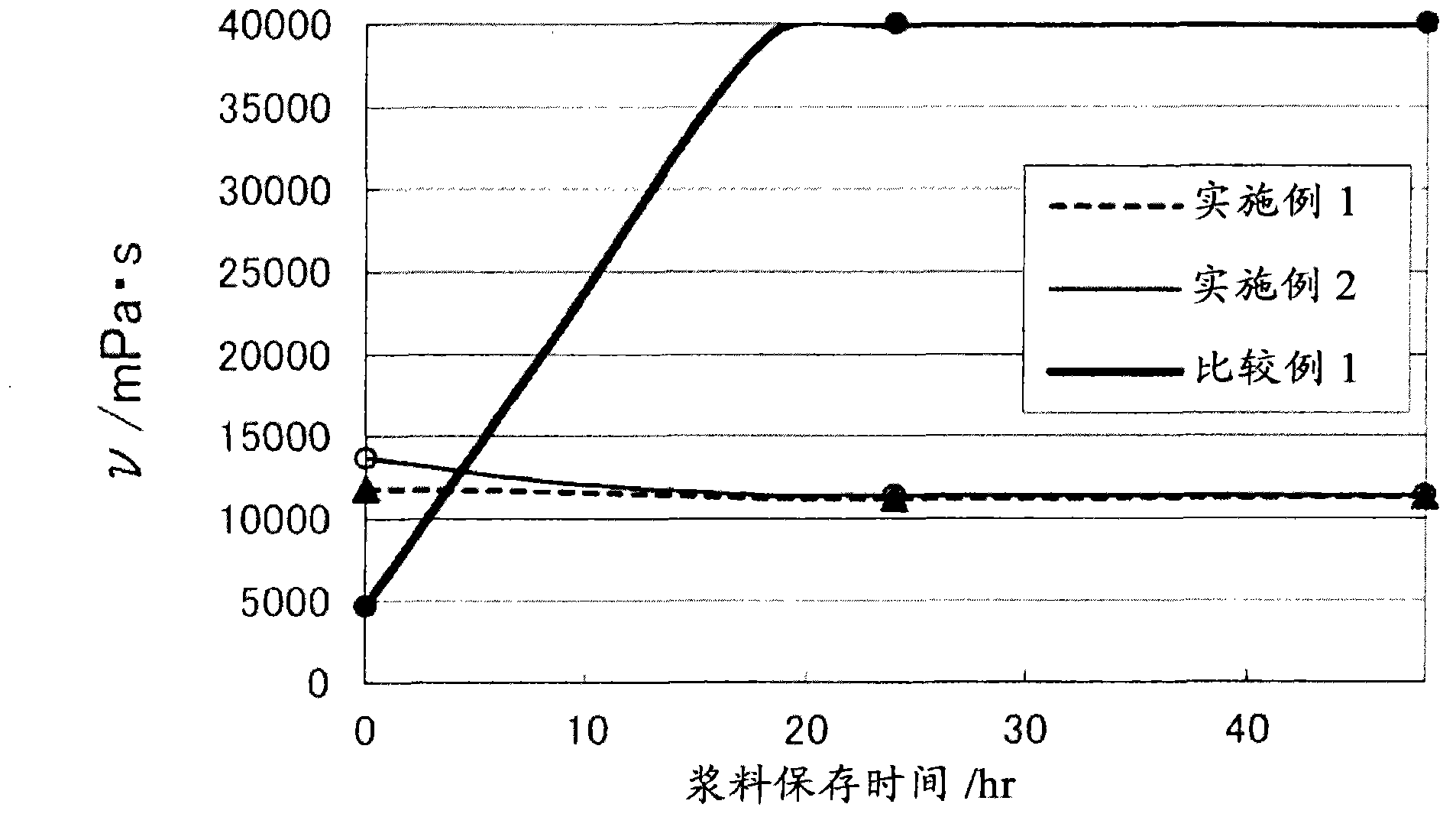
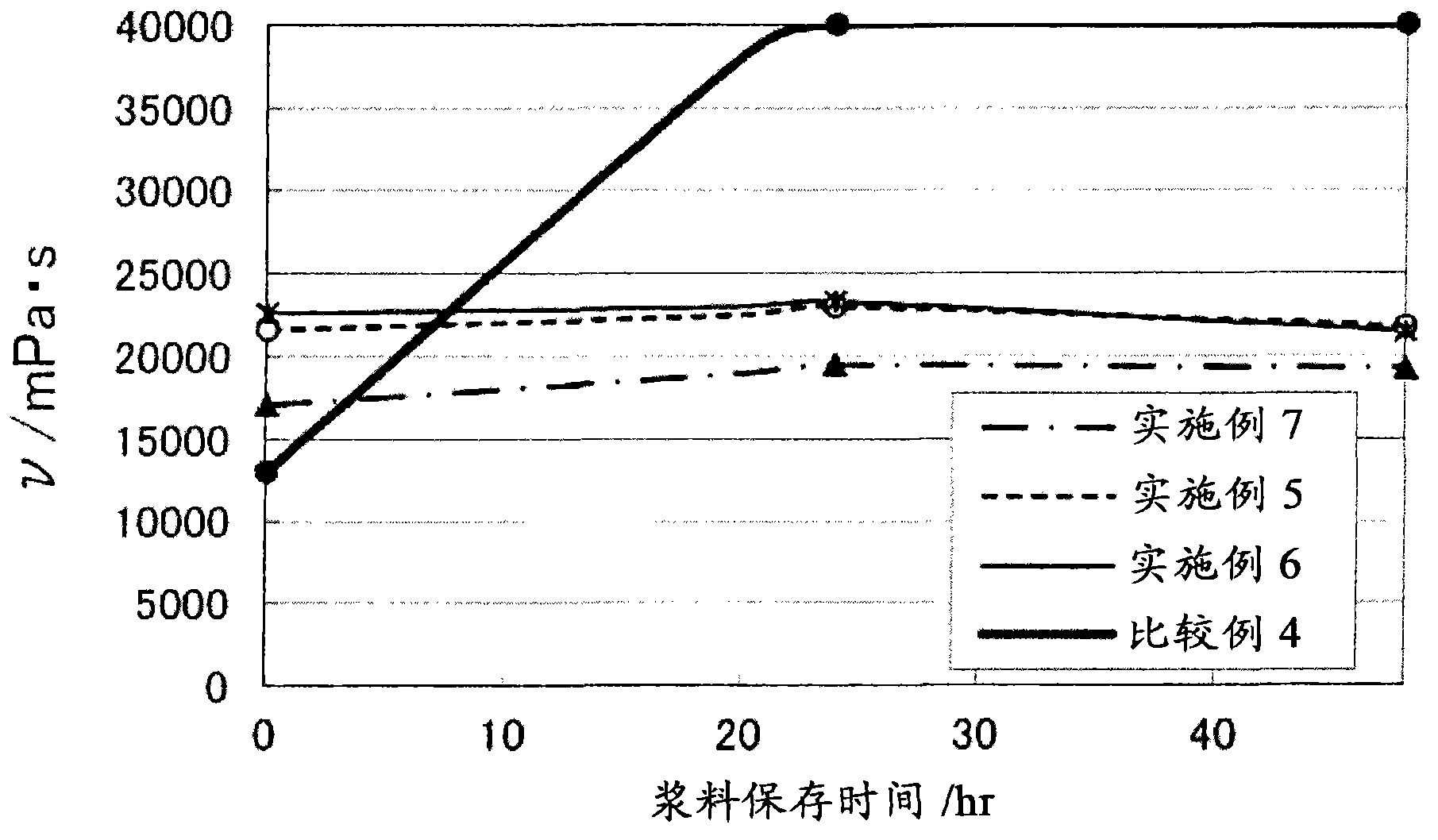
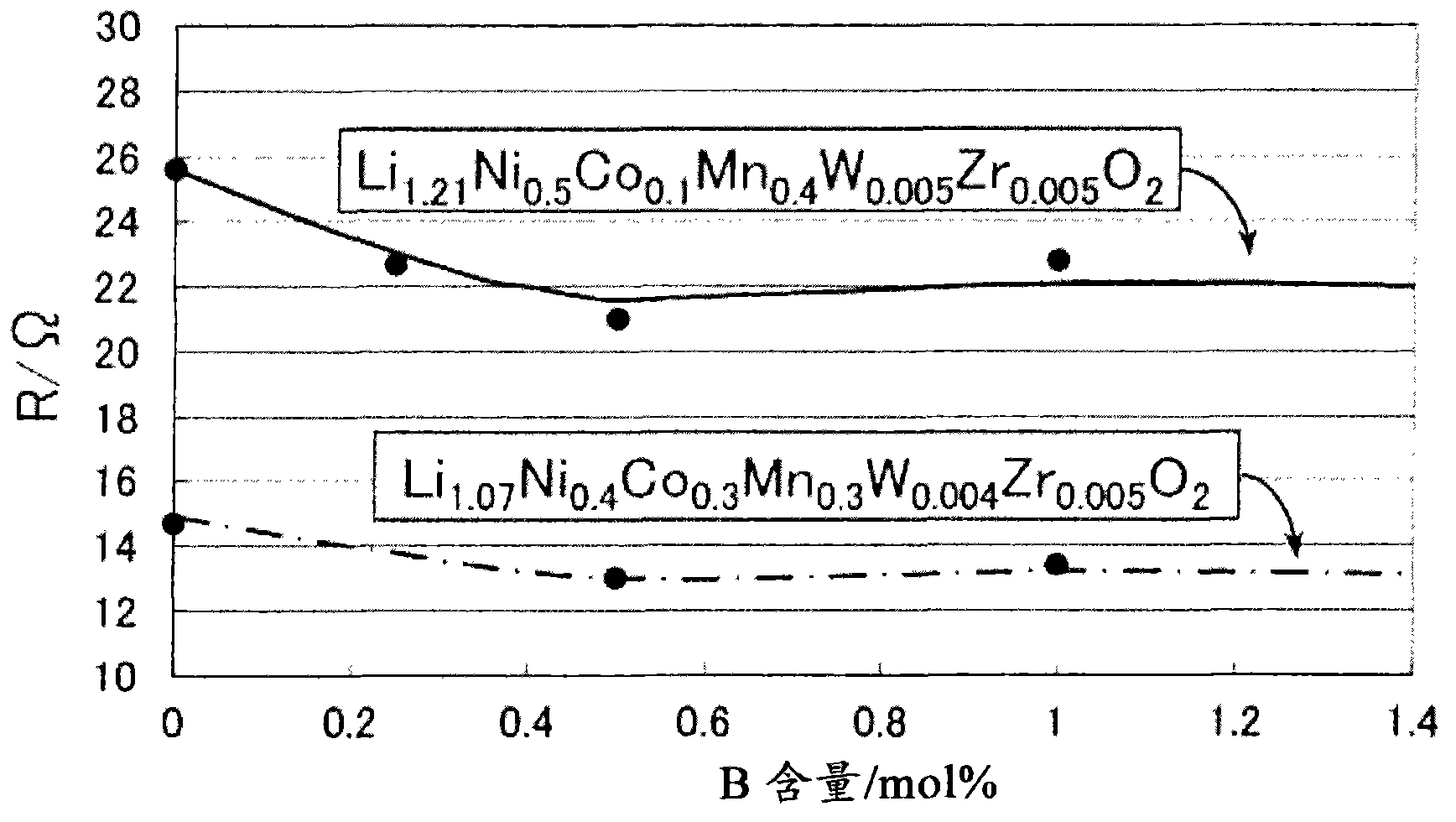Positive electrode composition for nonaqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, which is applied in the direction of non-aqueous electrolyte battery electrodes, secondary batteries, battery electrodes, etc., can solve the problems of undisclosed positive electrodes and lithium transition metal composite oxides, and achieve output characteristics and cycle Effects of property improvement and viscosity increase suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Prepare pure water in a stirred state in the reaction tank, and drop each aqueous solution of nickel sulfate, cobalt sulfate, and manganese sulfate at a flow ratio of Ni:Co:Mn=4:3:3 with the molar ratio of nickel, cobalt, and manganese. add. After the dropwise addition, the temperature of the solution was brought to 50° C., and then an aqueous sodium hydroxide solution was added dropwise quantitatively to obtain a nickel-cobalt-manganese composite hydroxide precipitate. Wash the obtained precipitate with water, filter, separate, and mix with lithium carbonate, tungsten oxide (VI) and zirconium oxide (IV), so that Li: (Ni+Co+Mn):W:Zr=1.07:1:0.004: 0.005 (molar ratio), to obtain mixed raw materials. The obtained mixed raw material was fired at 885° C. for 15 hours in an air atmosphere to obtain a sintered body. The obtained sintered body is pulverized and passed through a dry sieve to obtain the composition formula Li 1.07 Ni 0.4 co 0.3 mn 0.3 W 0.004 Zr 0.005 o ...
Embodiment 2
[0091] Except that the lithium transition metal composite oxide and boric acid (orthoboric acid) are mixed in such a manner that the ratio of the boron element to the lithium transition metal composite oxide is 1.0 mol%, the same operation as in Example 1 is performed to obtain Example 2. positive electrode composition.
Embodiment 3
[0093] In addition to mixing the precipitate of nickel-cobalt-manganese composite hydroxide with lithium carbonate, tungsten oxide (VI) and zirconium oxide (IV), Li: (Ni+Co+Mn):W:Zr=1.21:1:0.004: 0.005 (molar ratio), obtain with composition formula Li 1.21 Ni 0.4 co 0.3 mn 0.3 W 0.004 Zr 0.005 o 2 The cathode composition of Example 3 was obtained in the same manner as in Example 1 except for the lithium transition metal composite oxide shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com