Preparation method and application of a class of aniline quinazoline compounds
A technology of aniline quinazolines and compounds, which is applied in the field of aniline quinazoline compounds and their preparation, can solve the problems of low yield, long labeling time, short half-life, etc., achieve high specific activity of radioactivity, simple and convenient separation, Effects with simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
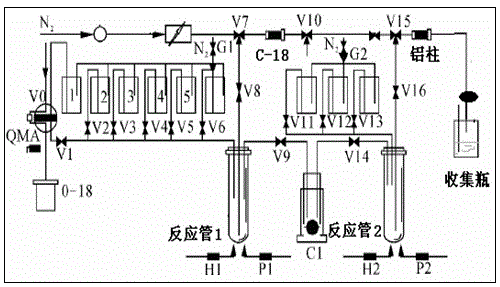
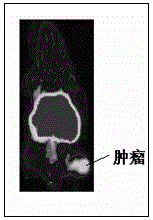
Image
Examples
Embodiment 1
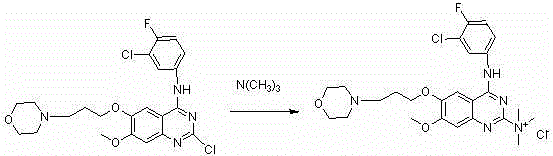
[0022] Example 1: Preparation of 6,7-dimethoxyquinazoline-4-aniline-2-trimethylamine chloride salt
[0023]
[0024] Dissolve 50 mg of 2-chloro-6,7-dimethoxyquinazoline-4-aniline in a mixed solvent of 3 mL of tetrahydrofuran and 1 mL of DMF, add 0.3 mL of trimethylamine in ethanol, and react under ice-cooling. After 5 days, the reaction was stopped, and a white solid was precipitated, which was filtered to obtain 20 mg. ESI-MS (m / z): 339 (M + ); 1 H NMR (DMSO- d 6 , 400 MHz) δ : 10.52 (1H, s, -NH-), 8.15 (1H, s, -5H), 7.73 (2H, d, J = 8.21 Hz, 2’-H, 6’-H), 7.45 (2H, d, J = 7.43 Hz, 3’-H, 5’-H), 7.26 (1H, s, 8-H), 7.21 (1H, t, J = 7.43 Hz, 5-H), 3.98 (6H, s, 2×OCH 3 ), 3.49 (9H, s, 3×NCH 3 ).
Embodiment 2
[0025] Example 2: Preparation of 6,7-dimethoxyquinazoline-4-(3'-bromo)aniline-2-trimethylamine chloride salt
[0026]
[0027] 63 mg of 2-chloro-6,7-dimethoxyquinazoline-4-(3'-bromo)aniline was dissolved in a mixed solvent of 3 mL of tetrahydrofuran and 1 mL of DMF, and 0.3 mL of trimethylamine in ethanol was added, React under ice bath. After 7 days, the reaction was stopped, and a white solid was precipitated, which was filtered to obtain 32 mg. ESI-MS (m / z): 418 (M + ); 1 H NMR (DMSO- d 6 , 400 MHz) δ : 10.52 (1H, s, -NH-), 8.15 (1H, s, 4’-H), 7.73 (1H, d, J = 8.21 Hz, 6’-H), 7.45 (1H, t, J = 7.43 Hz, 5'-H), 7.26 (1H, s, 8-H), 7.24 (1H, s, 5-H) 7.21 (1H, s, 2'-H), 3.98 (6H, s, 2 ×OCH 3 ), 3.49 (9H, s, 3×NCH 3 ).
Embodiment 3
[0028] Example 3: Preparation of 6,7-dimethoxyquinazoline-4-(3'-ethynyl)aniline-2-trimethylamine chloride salt
[0029]
[0030] 54 mg of 2-chloro-6,7-dimethoxyquinazoline-4-(3'-ethynyl)aniline was dissolved in a mixed solvent of 3 mL of tetrahydrofuran and 1 mL of DMF, and 0.3 mL of trimethylamine in ethanol was added , reacted in an ice bath. After 7 days, the reaction was stopped, and a white solid was precipitated, which was filtered to obtain 30 mg. ESI-MS (m / z): 363 (M + ); 1 H NMR (DMSO-d 6 , 400 MHz) δ : 10.52 (1H, s, -NH-), 8.15 (1H, s, 4’-H), 7.73 (1H, d, J = 8.21 Hz, 6’-H), 7.45 (1H, t, J = 7.43 Hz, 5'-H), 7.26 (1H, s, 8-H), 7.24 (1H, s, 5-H) 7.21 (1H, s, 2'-H), 3.98 (6H, s, 2 ×OCH 3 ), 3.49 (9H, s, 3×NCH 3 ), 3.09 (1H, s, alkyne hydrogen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com