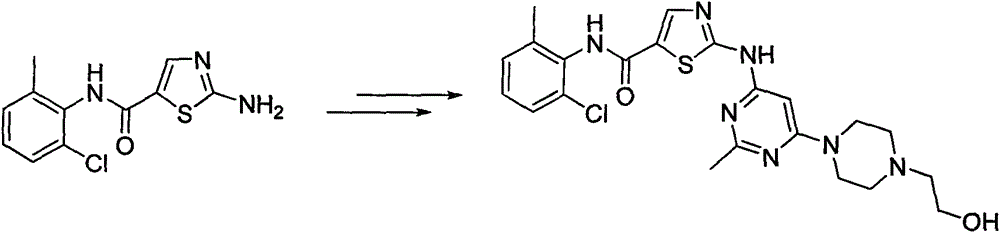
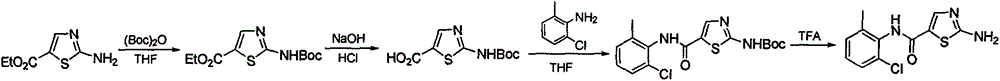A kind of synthetic method of 2-amino-n-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
A technology of methyl phenyl and synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of short synthesis route, high cost, and not amplified production synthesis process, and achieve short synthesis route, low equipment requirements and large implementation value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Synthesis of 3-ethoxyacrylic acid by one-pot method of alkali hydrolysis after nucleophilic substitution of trichloroacetyl chloride and vinyl ether, the specific method is:
[0037] Add 2kg of trichloroacetyl chloride into a 3L reaction bottle, cool with ice salt to below -5°C under stirring, then add 1kg of vinyl ether dropwise, and control the internal temperature <0°C. After the dropwise addition, keep the temperature < 0°C and react for 24 hours, then naturally return to temperature and stir for about 24 hours. GC detects that there is no trichloroacetyl chloride. Water pump vacuum distillation, the temperature of the reaction solution is gradually raised to 100°C, and the reaction solution is distilled until no gas is produced. Cool down to obtain a black liquid crude product, which is directly used in the next step.
[0038] Into a 10L reaction flask, add 500g of sodium hydroxide and 4L of water, stir to dissolve, and then cool down to room temperature. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com