Novel diazonaphthoquinone sulfonate derivative
A technology of diazonaphthoquinone sulfonate and derivatives of pimaric acid imide, which is applied in the field of new diazonaphthoquinone sulfonate derivatives, and can solve problems such as poor contrast and development tolerance, and limit practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
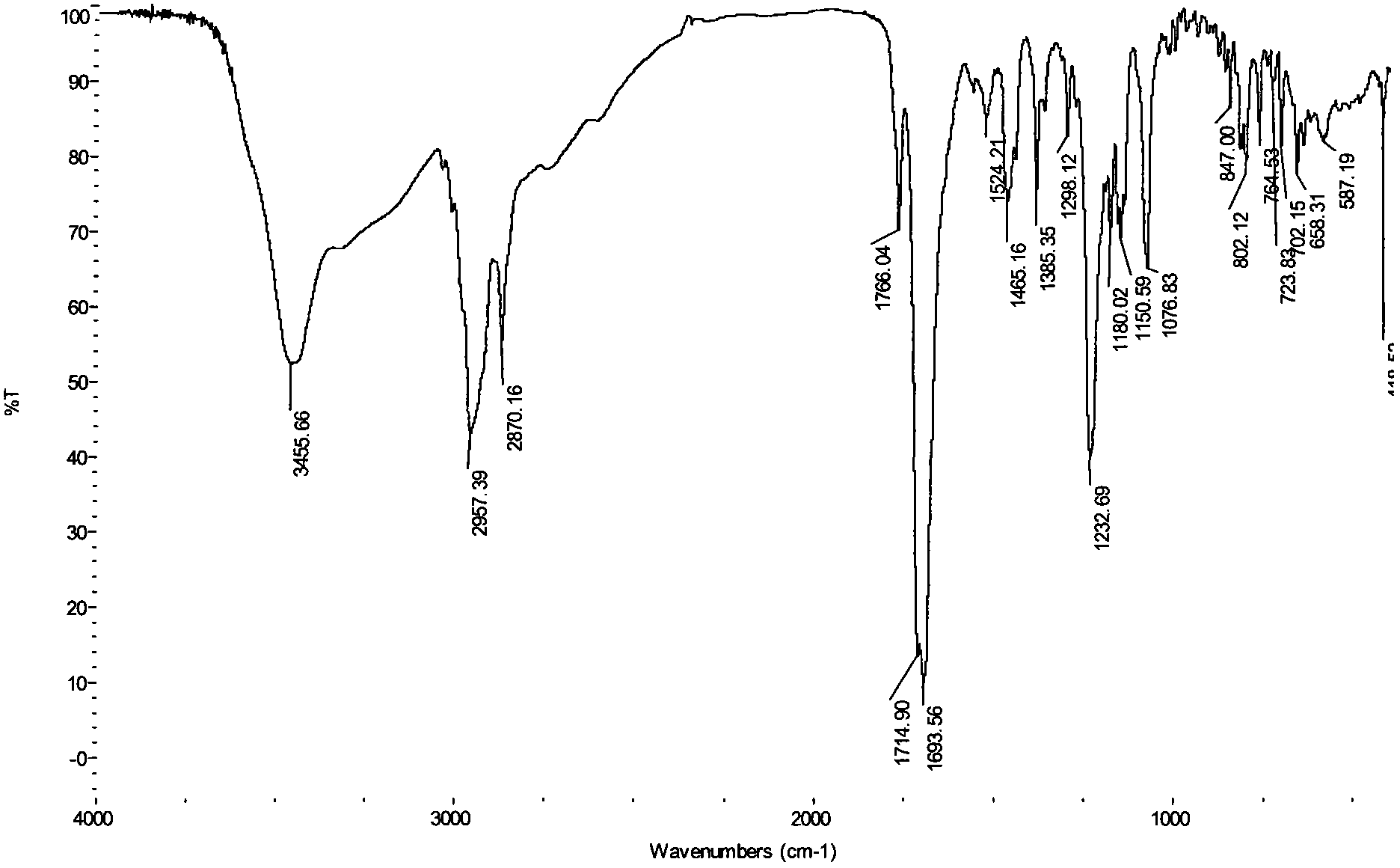
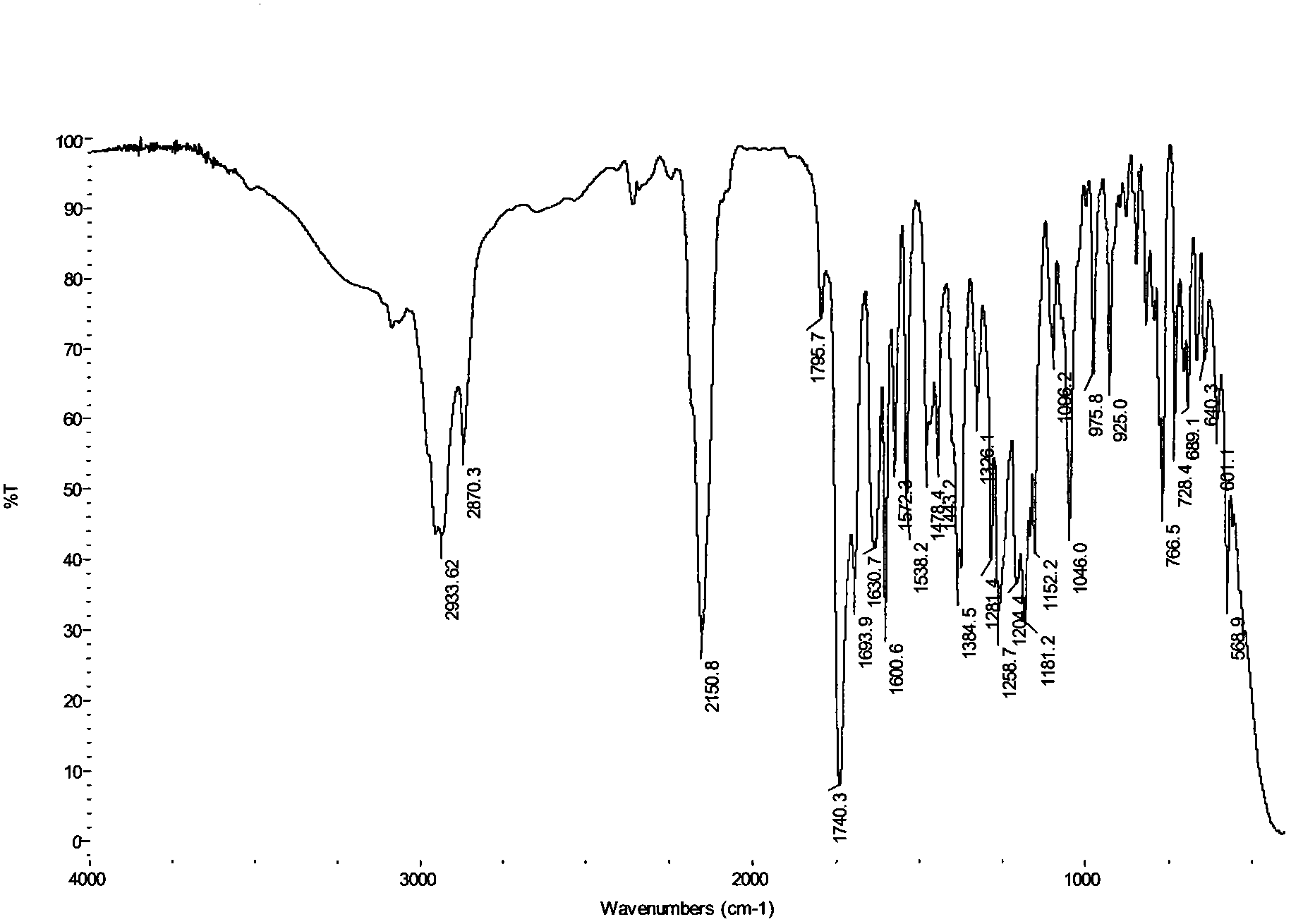
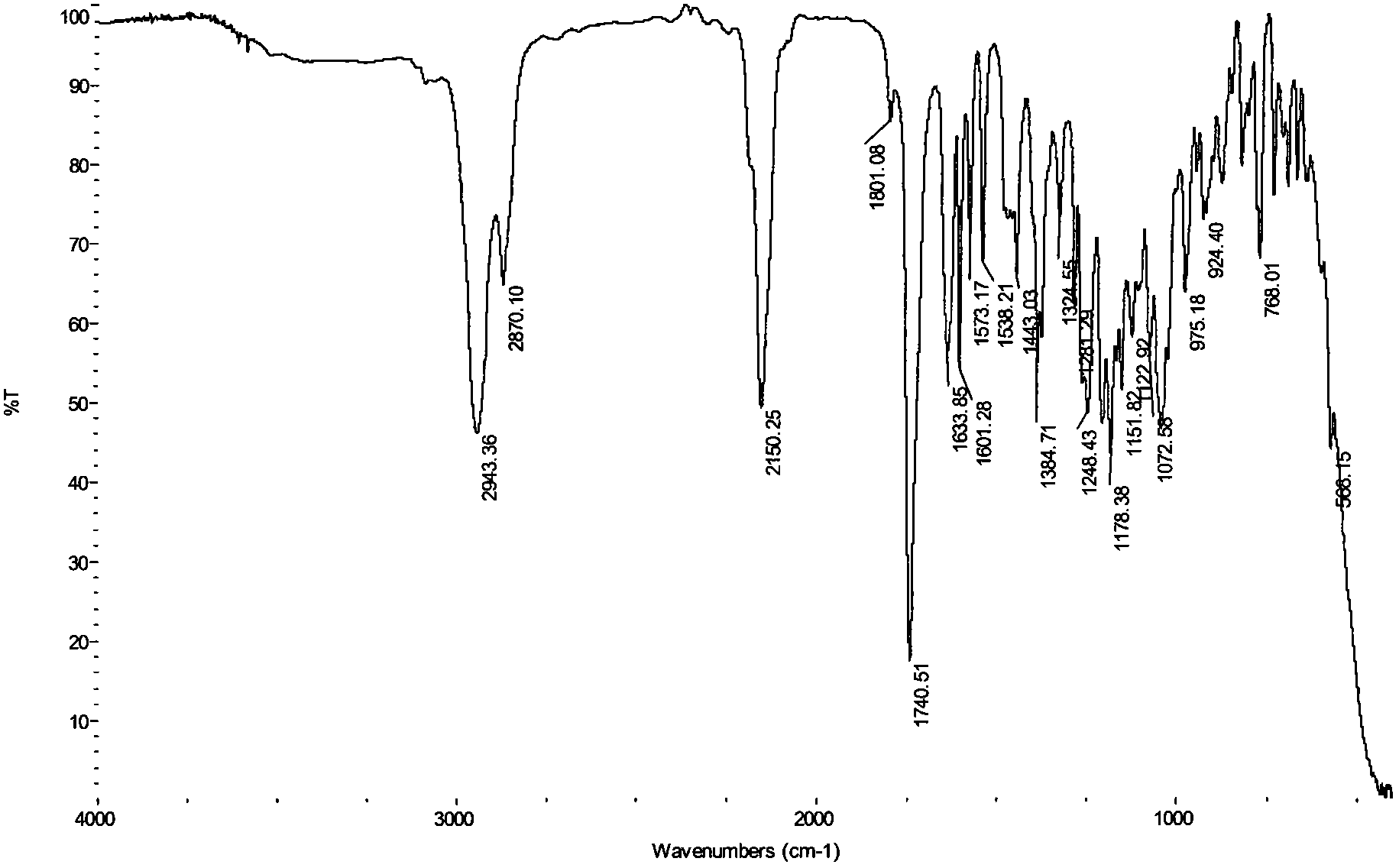
[0019] In the present invention, the parent compound N-hydroxymaleopimaric acid imide used can be prepared by the method described in our previous patent application (N-hydroxymaleopimaric acid imide sulfonate photoacid generator And its synthesis method, ZL: 200410039318.7), firstly obtained by reacting rosin with maleic anhydride and then reacting with hydroxylamine. Its structural formula is as follows:
[0020]
[0021] In the method of the present invention, the diazonaphthoquinone sulfonyl chloride used includes 2,1,4-diazonaphthoquinone sulfonyl chloride, 2,1,5-diazonaphthoquinone sulfonyl chloride or 2,1,6-diazonaphthalene sulfonyl chloride Quinone sulfonyl chloride. In terms of low price and easy availability, 2,1,4-diazonaphthoquinone sulfonyl chloride and 2,1,5-diazonaphthoquinone sulfonyl chloride are preferred, both of which are industrial products. Since 2,1,4-diazonaphthoquinone sulfonate has certain characteristics of generating acid after light exposure, which i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com