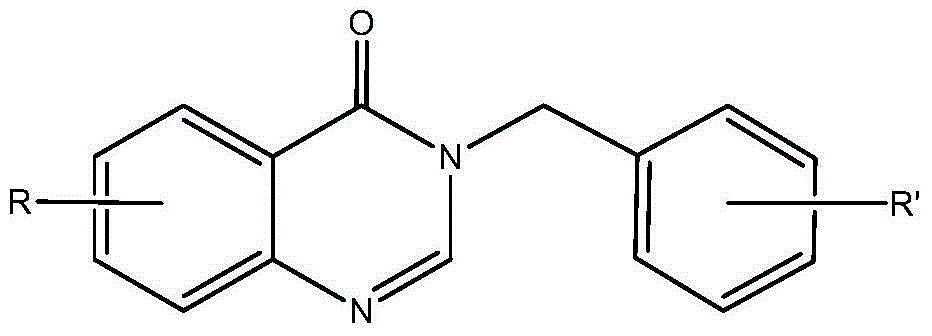
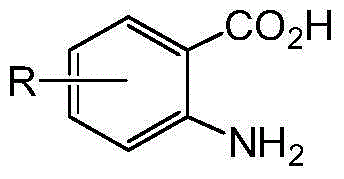
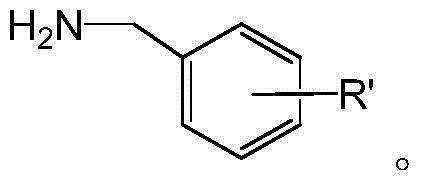
3-benzyl-4-quinazolinone compound and its synthesis method and application
A quinazolinone and compound technology, applied in the field of 3-benzyl-4-quinazolone compounds and their synthesis, can solve the problems of short action time, high production cost, weak drug effect, etc., and reduce shrinkage pressure, the synthesis method is easy, and the effect achieved by the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13-(4
[0055] Preparation of Example 13-(4-methoxybenzyl)-7-fluoro-4-quinazolinone (1)
[0056]Add absolute ethanol (10mL), 2-amino-4-fluorobenzoic acid (0.23g), 4-methoxybenzylamine (0.20g), triethyl orthoformate (0.6mL) into a 50mL round bottom flask , the mixture was stirred and refluxed for 5 h under the protection of nitrogen, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (chloroform:methanol=50:1). 1 HNMR (CDCl 3 ,400MHz),δ:3.79(3H,s,OCH 3 ),5.12(2H,s,Ar-CH 2 ), 6.88(2H,d,J=7.6Hz,Ar-H),7.22(1H,t,J=8.4Hz,Ar-H),7.30(1H,s,Ar-H),7.34(2H,d , J=10.0Hz, Ar-H), 8.11 (1H, s, Ar-H), 8.33 (1H, t, J=8.0Hz, Ar-H).
Embodiment 23-(4
[0057] Example 2 Preparation of 3-(4-methoxybenzyl)-7-chloro-4-quinazolinone (2)
[0058] 2-amino-4-chlorobenzoic acid was used instead of 2-amino-4-fluorobenzoic acid, isopropanol was used instead of absolute ethanol, and the reflux reaction time was 8 hours. The rest of the operations were the same as in Example 1. 1 HNMR (CDCl 3 ,400MHz),δ:3.79(3H,s,OCH 3 ),5.12(2H,s,Ar-CH 2 ),6.88(2H,d,J=7.6Hz,Ar-H),7.31(2H,d,J=7.6Hz,Ar-H),7.45(1H,d,J=8.4Hz,Ar-H), 7.71 (1H, s, Ar-H), 8.28 (2H, d, J=8Hz, Ar-H).
Embodiment 33-(4
[0059] Example 3 Preparation of 3-(4-methoxybenzyl)-6-bromo-4-quinazolinone (3)
[0060] 2-amino-5-bromobenzoic acid was used instead of 2-amino-4-fluorobenzoic acid, 2-methoxyethanol was used instead of ethanol, the reflux reaction time was 3 hours, and the rest of the operations were the same as in Example 1. 1 HNMR (CDCl 3 ,400MHz),δ:3.81(3H,s,OCH 3 ),5.16(2H,s,Ar-CH 2 ),6.90(2H,d,J=8.0Hz,Ar-H),7.33(2H,d,J=8.0Hz,Ar-H),7.62(1H,d,J=8.4Hz,Ar-H), 7.85 (1H, d, J=8.8Hz, Ar-H), 8.16 (1H, s, Ar-H), 8.45 (1H, s, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com