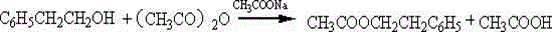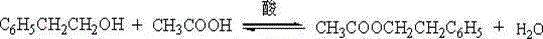Phenylethyl acetate synthesis method
A technology of phenylethyl acetate and a synthetic method, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of carbon-based compounds, can solve environmental problems, low yields, waste of resources, etc., and achieve low cost, Reduce energy consumption and production costs, easy to separate the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
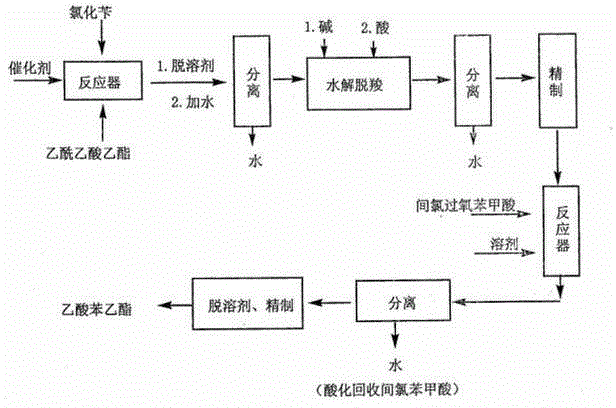
[0024] In a reaction flask equipped with a stirrer, a reflux condenser, a dropping funnel, and a thermometer, add 42 g of a sodium methoxide solution catalyst with a concentration mass / mass of 30%, and 31 g of ethyl acetoacetate, and stir for 15 minutes. Heat to reflux, add 25.3g of benzyl chloride dropwise, add about 1h, and reflux for 2h to generate ethyl benzyl acetoacetate. Cool to room temperature, add 100ml of water to dissolve and stir well, adjust the pH to 7 with concentrated hydrochloric acid, let it stand for stratification, separate the oily substance in the lower layer, wash with water, and let it stand for stratification again to obtain the product ethyl benzyl acetoacetate.
[0025] Add 60 grams of 5% sodium hydroxide solution to the above product to make the solution alkaline, reflux for 2 hours under stirring, oily matter is formed, cool to below 40°C and add concentrated hydrochloric acid to adjust the pH to 1-2. Continue heating to reflux for 1.5h. The dist...
Embodiment 2
[0028] In a reaction flask equipped with a stirrer, a reflux condenser, a dropping funnel, and a thermometer, add 45 g of a sodium methoxide solution catalyst with a concentration mass / mass of 28%, and 31 g of ethyl acetoacetate, and stir for 15 minutes. Heat to reflux, add 25.3g of benzyl chloride dropwise, add about 1h, and reflux for 3h to generate ethyl benzyl acetoacetate. Cool to room temperature, add 100ml of water to dissolve and stir well, adjust the pH to 7 with concentrated hydrochloric acid, let it stand for stratification, separate the oily substance in the lower layer, wash with water, and let it stand for stratification again to obtain the product ethyl benzyl acetoacetate.
[0029] Add 60 grams of 5% sodium hydroxide solution to the above product to make the solution alkaline, reflux for 3 hours under stirring, oily matter is formed, cool to below 40°C and add concentrated hydrochloric acid to adjust the pH to 1-2. Continue heating to reflux for 2h. The distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com