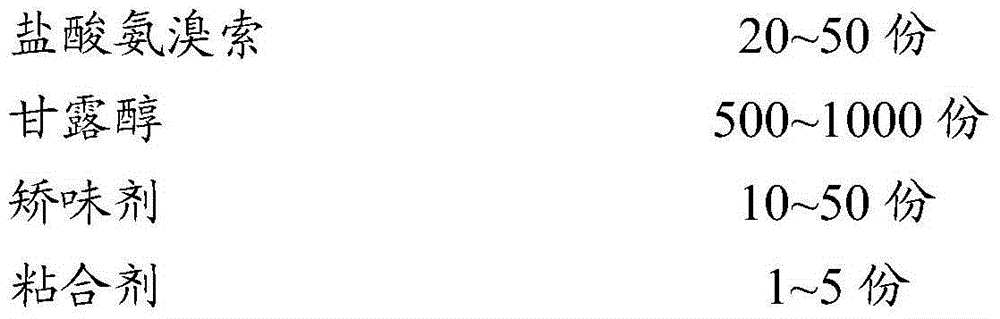Ambroxol hydrochloride granules and preparation method thereof
A technology of ambroxol hydrochloride and granules, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can cover the bad smell and taste, avoid the sticking phenomenon, and reduce the phenomenon of moisture absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Pass the raw material medicine and auxiliary materials through a 100-mesh sieve respectively, and set aside.
[0030] Weigh the raw materials of the following prescription quantities:
[0031]
[0032] Weigh 0.02g of hypromellose (model: HT-K, viscosity: 4000mPa.s) and 0.03g of acrylic resin No. II (manufacturer: Xi'an Zaolotang Pharmaceutical Group Rehabilitation Medicine Co., Ltd., medicinal) in Slowly add distilled water under constant stirring to make a 2% water dispersion by mass, and put it in a spray tank for later use. Put 0.3g of ambroxol hydrochloride into the fluidized bed, the inlet air temperature is 60°C-80°C, the outlet air temperature is 50°C-70°C, and the feeding rate is 10ml / min, spray it, and pass through a 30-mesh sieve after forming microcapsules. Add 9 g of mannitol and 0.1 g of stevioside, spray and mix evenly, dry at 50-60° C., and sieve with 20 meshes for sizing to obtain 1,000 bags of ambroxol hydrochloride granules.
Embodiment 2
[0034] Pass the raw material medicine and auxiliary materials through a 100-mesh sieve respectively, and set aside.
[0035] Weigh the raw materials of the following prescription quantities:
[0036]
[0037] Weigh 0.02g of hypromellose (model: HT-K, viscosity: 6000mPa.s) and 0.03g of acrylic resin No. II (manufacturer: Xi'an Zaolotang Pharmaceutical Group Rehabilitation Medicine Co., Ltd., medicinal) in Slowly add distilled water under constant stirring to make a 2% water dispersion by mass, and put it in a spray tank for later use. Put 0.4g of ambroxol hydrochloride into the fluidized bed, the inlet air temperature is 60°C-80°C, the outlet air temperature is 50°C-70°C, and the feed rate is 10ml / min, spray it, and pass through a 30-mesh sieve after forming microcapsules. Add 8 g of mannitol and 0.1 g of stevioside, spray and mix evenly, dry at 50-60° C., and sieve through a 20-mesh sieve to obtain 1,000 bags of ambroxol hydrochloride granules.
Embodiment 3
[0039] Pass the raw material medicine and auxiliary materials through a 100-mesh sieve respectively, and set aside.
[0040] Weigh the raw materials of the following prescription quantities:
[0041]
[0042] Weigh 0.02g of hypromellose (model: HT-K, viscosity: 2000mPa.s) and 0.03g of acrylic resin No. II (manufacturer: Xi'an Zaolotang Pharmaceutical Group Rehabilitation Medicine Co., Ltd., medicinal) in Slowly add distilled water under constant stirring to make a 2% water dispersion by mass, and put it in a spray tank for later use. Put 0.5g of ambroxol hydrochloride into the fluidized bed, the inlet air temperature is 60°C-80°C, the outlet air temperature is 50°C-70°C, and the feeding rate is 10ml / min, spray it, and pass through a 30-mesh sieve after forming microcapsules. Add 8 g of mannitol and 0.2 g of stevioside, spray and mix evenly, dry at 50-60° C., sieve with 20 meshes for granulation, and obtain 1000 bags of ambroxol hydrochloride granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com