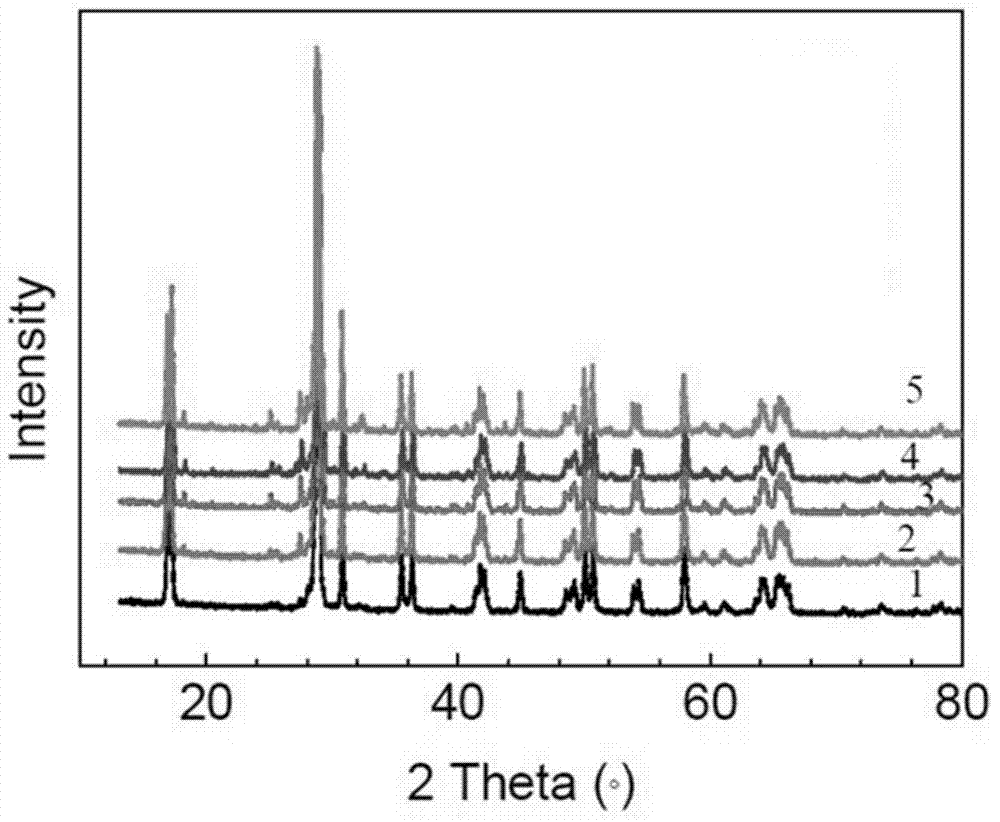
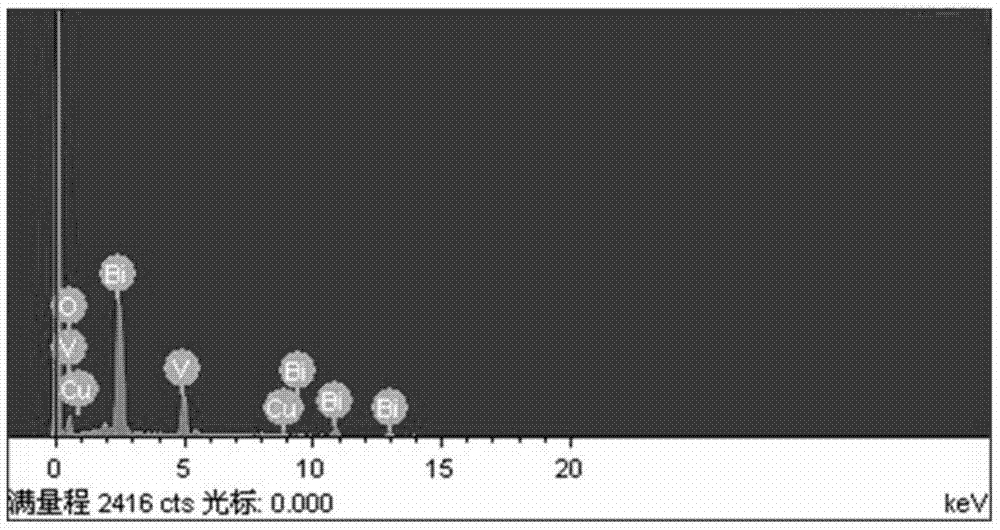
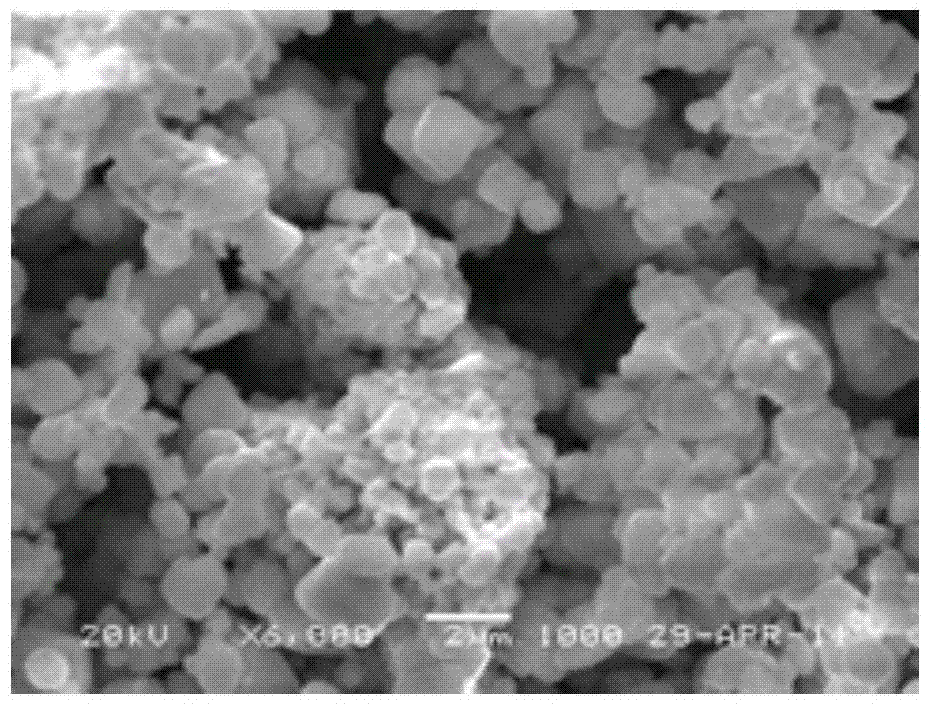
Method for preparing yellow bismuth vanadate pigment via molten salt method
A molten salt method and bismuth vanadate technology, applied in the field of inorganic pigments, can solve the problems of pigment color and luster, poor chemical stability, uneven pigment particle size distribution, and difficulty in fully carrying out the reaction, so as to shorten the calcination time, reduce the calcination temperature, Avoid the effects of the use of acids and bases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] ⑴ put 15.52g V 2 o 5 , 39.76g Bi 2 o 3 , 110.56g KNO 3 After ball milling for 30 minutes, the powder precursor was prepared.
[0026] (2) Heat the powder precursor obtained in step (1) in a muffle furnace at 500° C. for 3 hours in an air atmosphere.
[0027] (3) Naturally cool the material obtained in step (2) to room temperature, dissolve the nitrate in it with hot water, separate the solid sample by suction filtration, and then dry it at 100°C to obtain BiVO with monoclinic structure 4 product.
Embodiment 2
[0029] ⑴ put 9.09g V 2 o 5 , 23.30g Bi 2 o 3 After ball milling for 30 minutes, add 162g NaNO 3 After continuing ball milling for 10 minutes, a powder precursor was prepared.
[0030] (2) Heat the powder precursor obtained in step (1) in a muffle furnace at 400° C. for 9 hours in an air atmosphere.
[0031] (3) Naturally cool the material obtained in step (2) to room temperature, dissolve the nitrate in hot water, separate the solid sample by suction filtration, and then dry it at 110°C to obtain BiVO with monoclinic structure 4 product.
Embodiment 3
[0033] ⑴ put 9.09g V 2 o 5 , 23.30g Bi 2 o 3 After ball milling for 10 minutes, add 13.73g LiNO 3 and 18.66g KNO 3 , and continue ball milling for 10 minutes to make a powder precursor.
[0034] (2) Heat the powder precursor obtained in step (1) in a muffle furnace at 350° C. for 12 hours in an air atmosphere.
[0035] (3) Naturally cool the material obtained in step (2) to room temperature, dissolve the nitrate in hot water, separate the solid sample by suction filtration, and then dry it at 90°C to obtain BiVO with monoclinic structure 4 product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com