Glucomannan succinate preparation method
A technology of glucomannan and succinate, applied in the fields of cosmetics, medicines and health food
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of Baiji polysaccharide succinate described in this example: The preparation of raw materials such as Baiji polysaccharide 70 (BT70, Mw=70kDa) was carried out with reference to the patent literature (application number 201010166718.X). Weigh 10 g of the dried BT70 raw material respectively, put it in a 50 mL three-neck flask, add 15 mL of DMF, and stir to dissolve at 100°C. Add 3 mL of N,N-diisopropylethylamine at the set temperature, and add succinic anhydride according to the molar ratio of raw material monosaccharide group and succinic anhydride is 1:1, react for a certain period of time, and take samples regularly. Add 2 times the amount of absolute ethanol to the reaction solution to precipitate a precipitate, filter it with suction, wash with absolute ethanol 3-4 times, and dry the obtained white powder in an oven at 60°C to obtain a reaction product. The determination of the succinyl substitution degree refers to the literature method of Takahara et a...
Embodiment 2
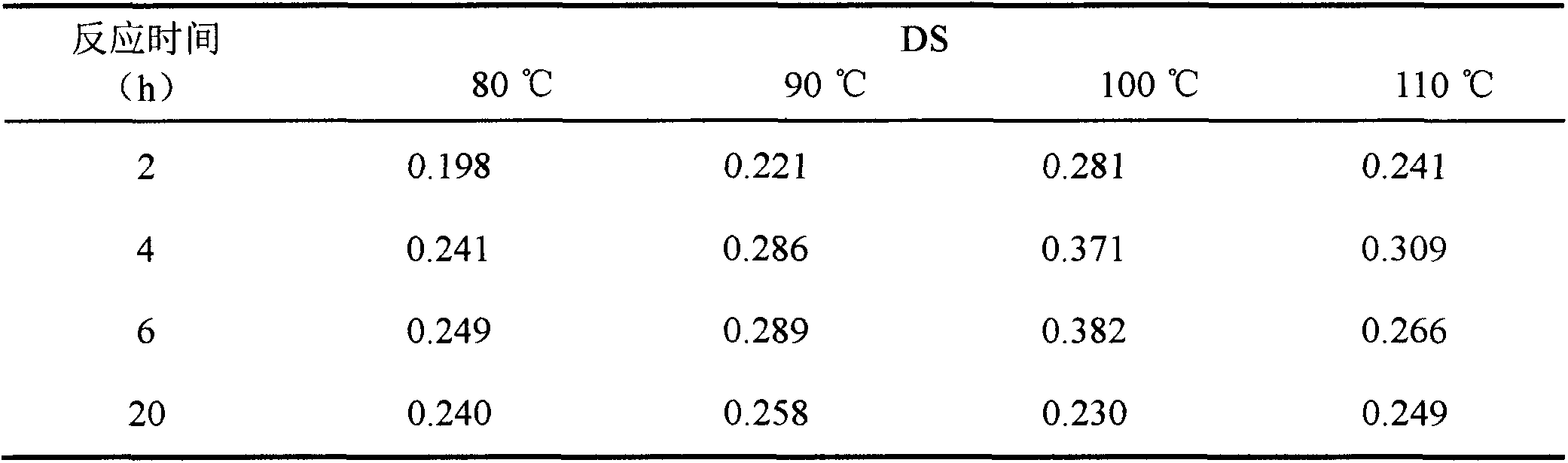
[0019] The preparation of the white and polysaccharide succinate described in this example: Weigh 1.0 g of dried BT70 raw materials respectively, adopt a reaction temperature of 100° C., except that the molar ratio of BT70 and succinic anhydride is changed to 1:0.5, 1:1, Except for 1:3, 1:5, and 1:10, the rest of the operations refer to Example 1. The influence of the molar feed ratio of BT70 and succinic anhydride on the reaction was investigated, and the measurement results are shown in Table 2.
[0020] The influence of table 2 reaction raw material mol ratio on the degree of substitution of BT70 succinyl ester
[0021]
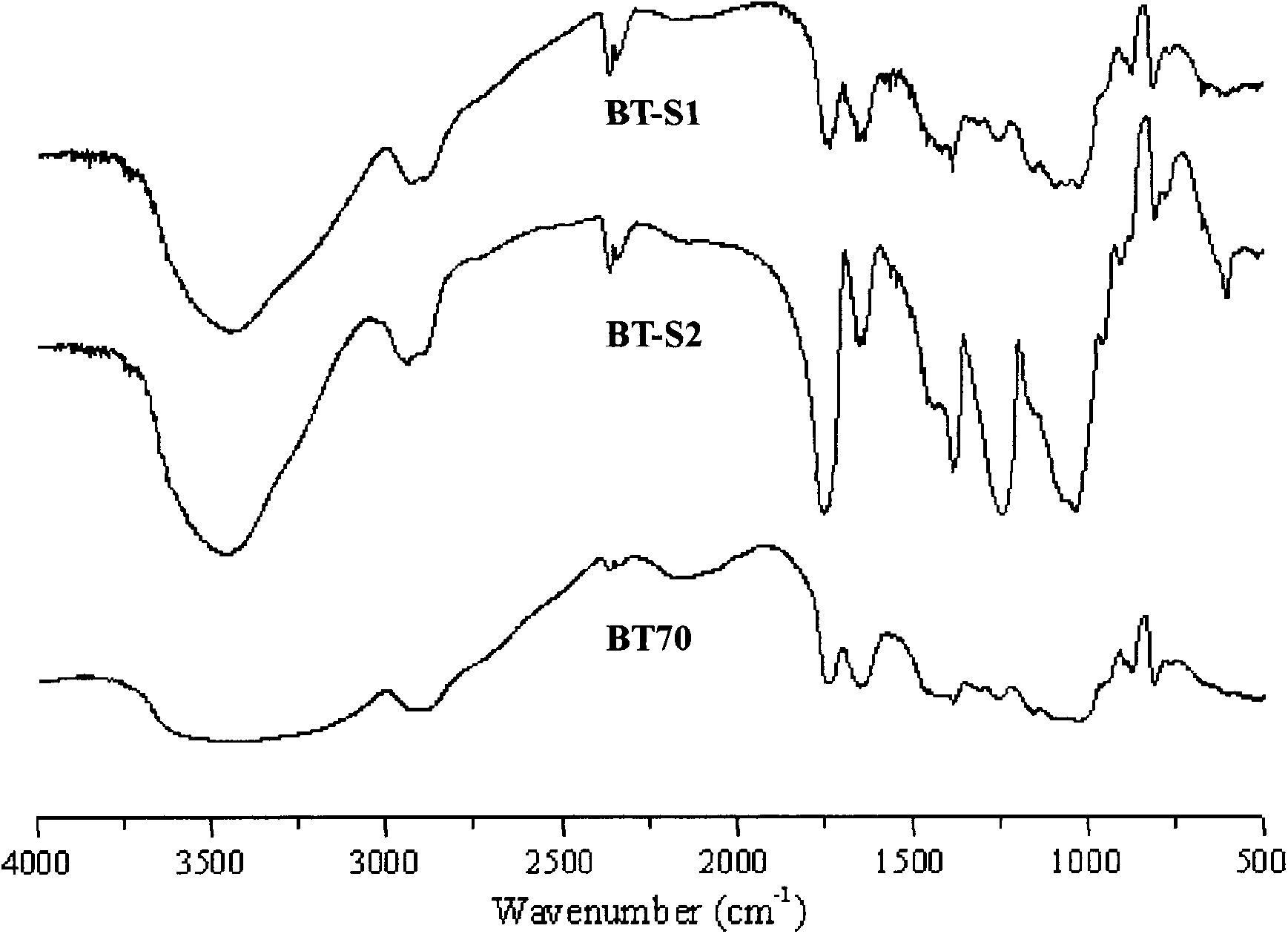
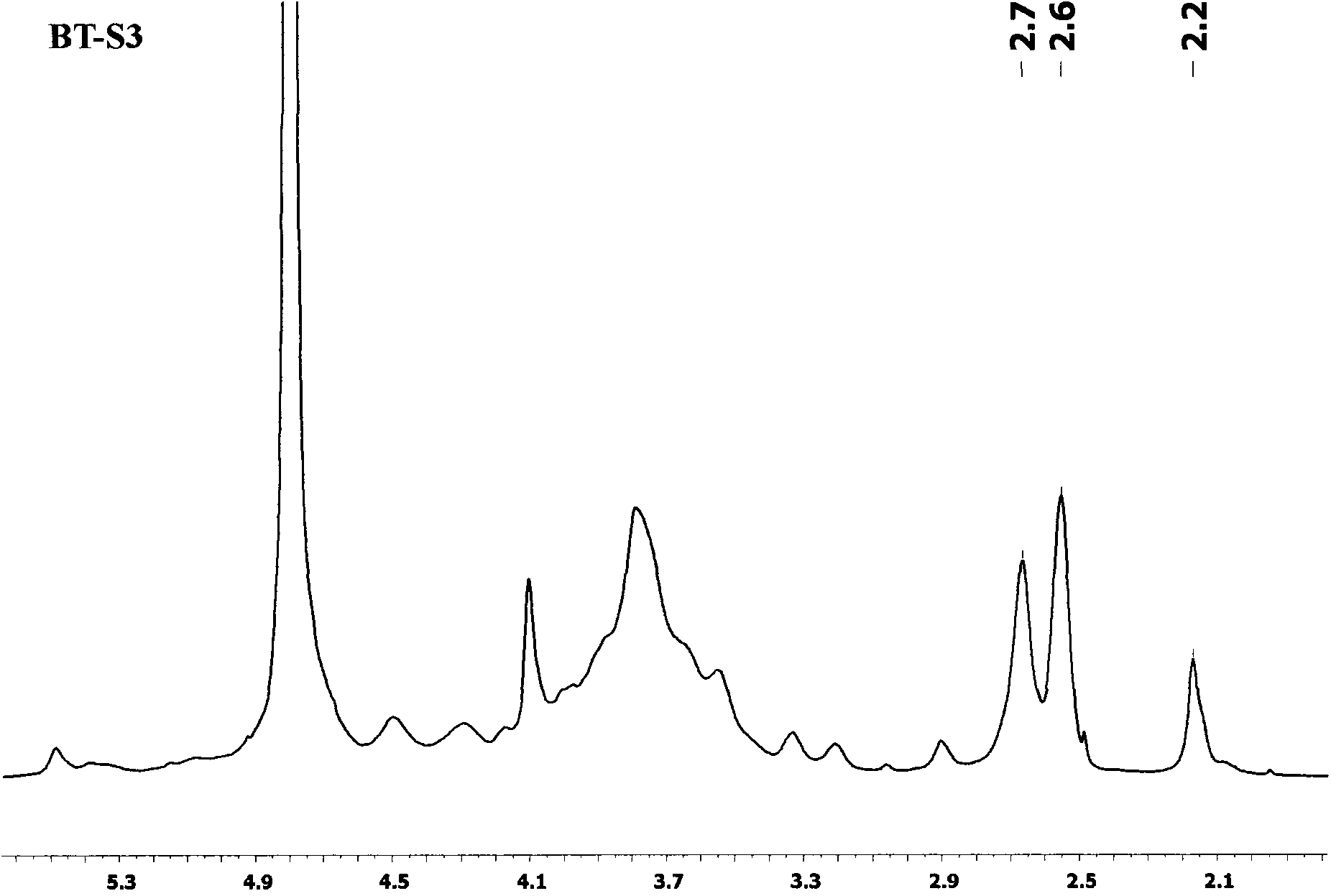
[0022] Structural testing of reaction products: (1) Infrared spectrum analysis results show that BT70 and its succinate reaction products BT70-S1 (DS0.38), BT70-S2 (DS0.85) etc. are at 1750cm -1 The corresponding carbonyl characteristic absorption peak in the vicinity is obviously enhanced, see the attached figure 1 (2) H NMR analysis results show tha...
Embodiment 3
[0025] Preparation of glucomannan succinate described in this example: Weigh dry BT40 (1.0g), BT70 (1.0g), BT180 (0.5g), konjac polysaccharide (0.2g) and aloe polysaccharide (0.5g) respectively g) Raw materials, the molar ratio of polysaccharide to succinic anhydride is 1:10, the reaction temperature is 100°C, the reaction time is 6h, and the rest of the operations are carried out with reference to Example 1. The preparation of different glucomannan succinic anhydride was investigated respectively, and the determination results are shown in Table 3.
[0026] Table 3 Degree of substitution of different glucomannan succinyl esters
[0027]
[0028] Conclusion: The corresponding succinyl ester products of different glucomannans can be obtained by using the above reaction method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com