Preparation method of multi-substituted fused ring compounds
A multi-substitution, compound technology, applied in the field of preparation of functional materials, can solve the problems of expensive substrates and environmentally unfriendly by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of N-(5,6,7,8-tetraphenylnaphthalen-1-yl)pivalamide (3a)
[0037]
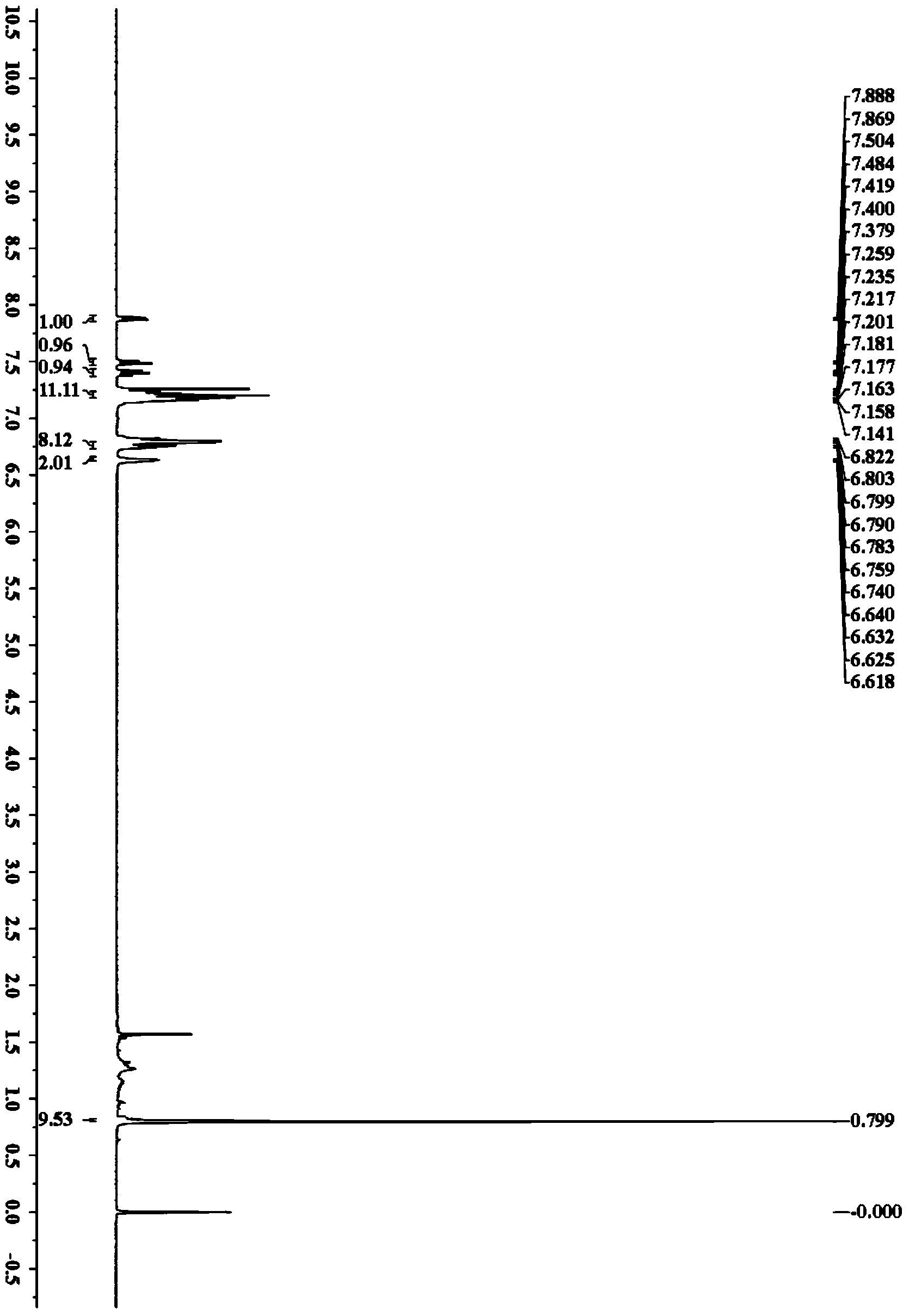
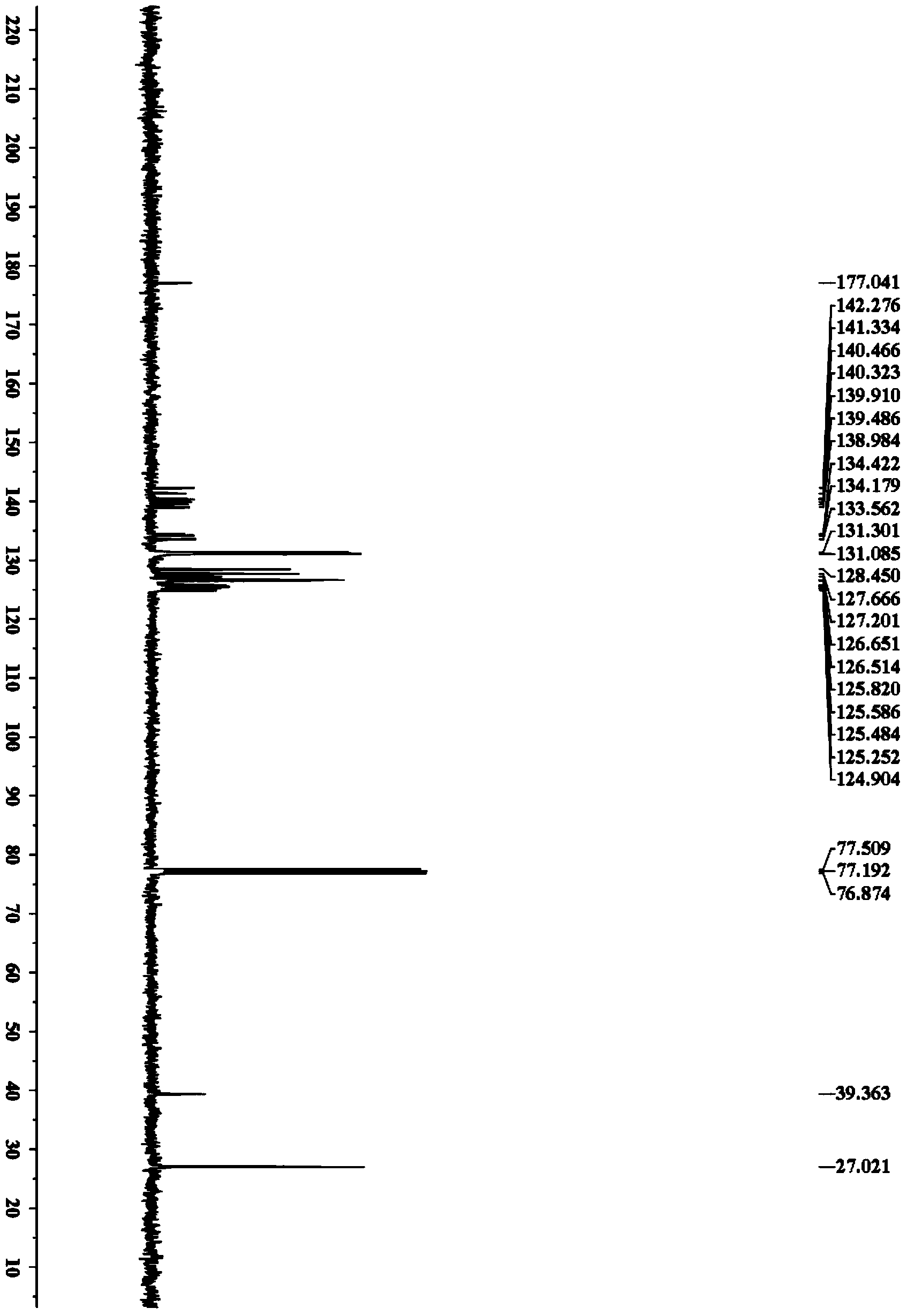
[0038] Accurately weigh N-pivaloylanilide (44.3mg, 0.25mmol), rhodium chloride (2.6mg, 0.0125mmol), toluene (89.2mg, 0.5mmol), copper acetate (45.5mg, 0.25mmol), and Sequentially added to a 25mL Schlenk bottle, added refined ethanol (3.0mL), and placed in an oil bath at 50°C for 8h. After the reaction, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent, and the silica gel column was used for separation. The yield of N-(5,6,7,8-tetraphenylnaphthalen-1-yl)pivalamide was 80%. 1 H NMR (400MHz, CDCl 3 )δ7.88(d,J=7.6Hz,1H),7.49(d,J=8.2Hz,1H),7.40(dd,J=7.6,8.2Hz,1H),7.24-7.14(m,11H), 6.82-6.74(m,8H),6.64-6.62(m,2H),0.80(s,9H); 13 C NMR (100MHz, CDCl 3 )δ177.0,142.3,141.3,140.5,140.3,139.9,139.5,139.0,134.4,134.2,133.6,131.3,131.1,128.5,127.7,127.2,126.7,126.5,125.8,125.6,125.5,125.3,124.9,39.4,27.0 ;IR(KBr)υ(cm -1 )3425,3337,30...
Embodiment 2
[0039] Example 2: Synthesis of N-(5,6,7,8-tetrakis(4-fluorophenyl)naphthalen-1-yl)pivalamide (3b)
[0040]
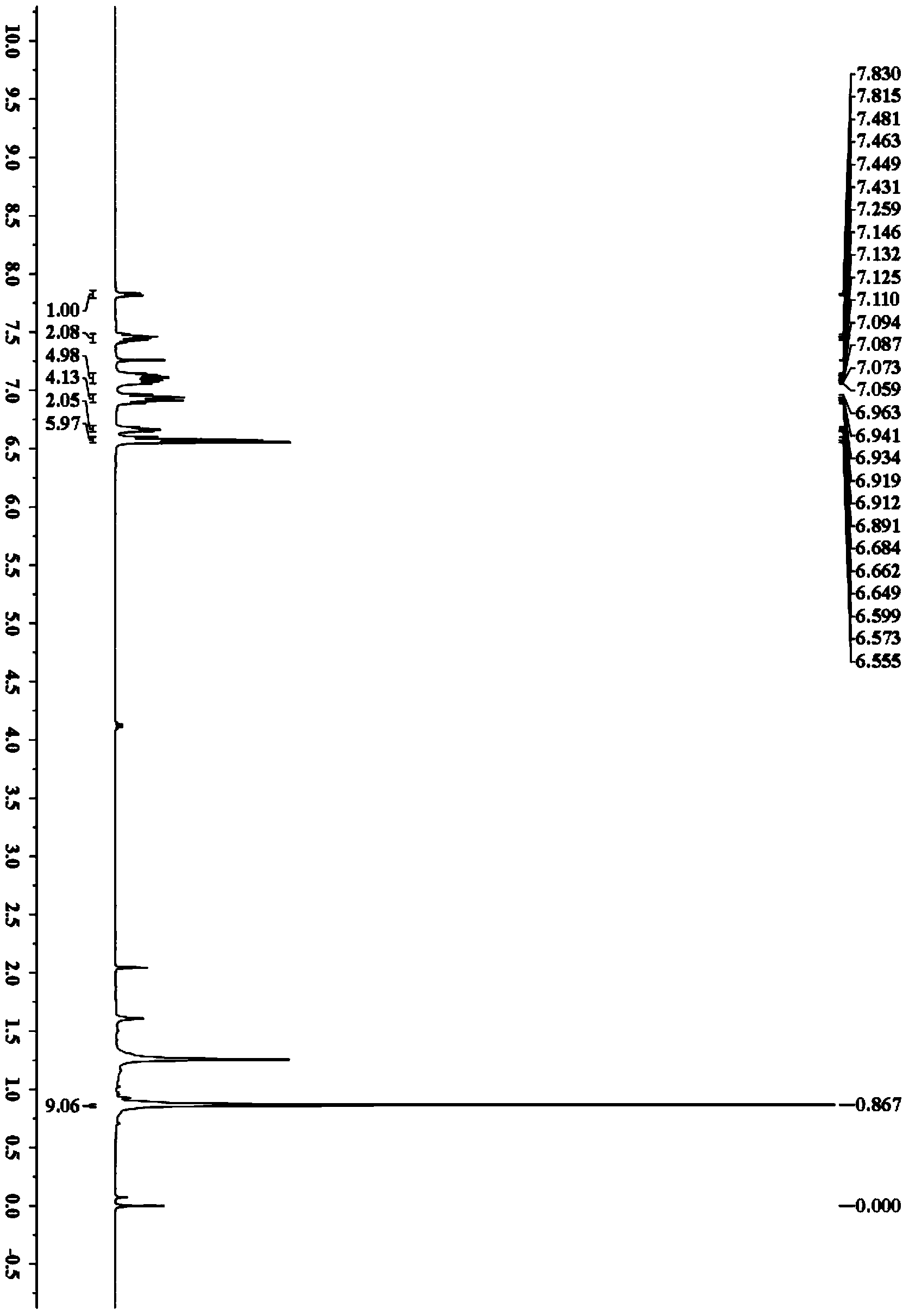
[0041] Accurately weigh N-pivaloylanilide (44.3mg, 0.25mmol), rhodium acetate (1.1mg, 0.0025mmol), 1,2-di(4-fluorophenyl)acetylene (107.0mg, 0.5mmol), iodide Cuprous (47.6mg, 0.25mmol) was added to a 25mL Schlenk bottle in turn, and purified cyclohexane (3.0mL) was added, and placed in an oil bath at 100°C for 10h. After the reaction was over, the solvent was removed under reduced pressure, using petroleum ether / ethyl acetate as eluent, separated on a silica gel column, N-(5,6,7,8-tetrakis(4-fluorophenyl)naphthalen-1-yl)pivalamide The yield was 63%. 1 H NMR (400MHz, CDCl 3)δ7.82(d,J=6.2Hz,1H),7.48-7.43(m,2H),7.13-7.07(m,5H),6.96-6.89(m,4H),6.68-6.65(m,2H) ,6.60-6.56(m,6H),0.87(s,9H); 13 C NMR (100MHz, CDCl 3 )δ176.9,161.8(d, 1 J C–F =246.7Hz), 161.6(d, 1 J C–F =244.7Hz), 160.7(d, 1 J C–F =243.9Hz), 160.6(d, 1 J C–F =244.2Hz),140.4,138.9,138.2,137.81,137....
Embodiment 3
[0042] Example 3: Synthesis of N-(5,6,7,8-tetrakis(4-chlorophenyl)naphthalen-1-yl)pivalamide (3c)
[0043]
[0044] Accurately weigh N-pivaloylanilide (44.3mg, 0.25mmol), dichloro(cyclopentadienyl) rhodium(III) dimer (2.4mg, 0.005mmol), 1,2-bis(4- Chlorophenyl) acetylene (123.5mg, 0.5mmol), cuprous bromide (17.9mg, 0.125mmol), and added to a 25mL Schlenk bottle in turn, added refined toluene (3.0mL), placed in 60 ° C oil Reaction in the bath for 7h. After the reaction was over, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as eluent, followed by silica gel column separation, N-(5,6,7,8-tetrakis(4-chlorophenyl)naphthalen-1-yl)pivalamide The yield was 76%. 1 H NMR (400MHz, CDCl 3 )δ7.76(dd,J=5.0,3.7Hz,1H),7.44-7.43(m,2H),7.26-7.18(m,4H),7.07(dd,J=10.0,8.4Hz,4H),6.92 (s,1H),6.89-6.86(m,4H),6.64(d,J=8.4Hz,2H),6.55(d,J=8.4Hz,2H),0.87(s,9H); 13 C NMR (100MHz, CDCl 3 )δ177.0,140.1,139.7,138.7,138.2,138.0,137.7,137.6,134.1,134.0,13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com