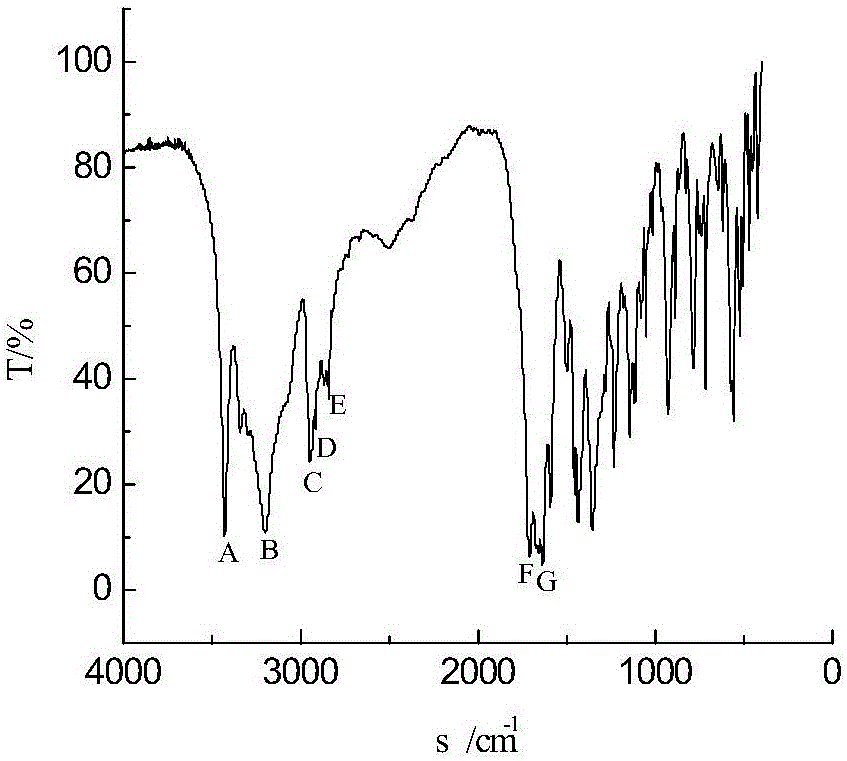
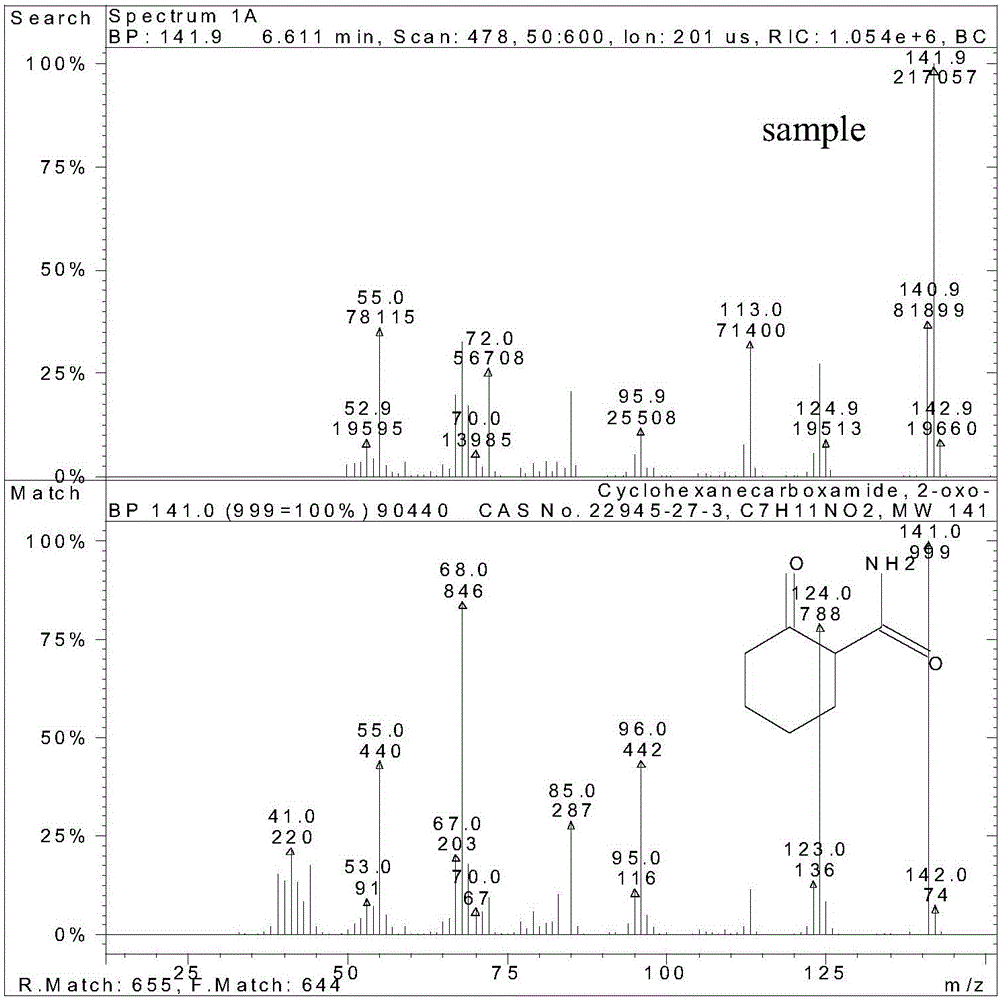
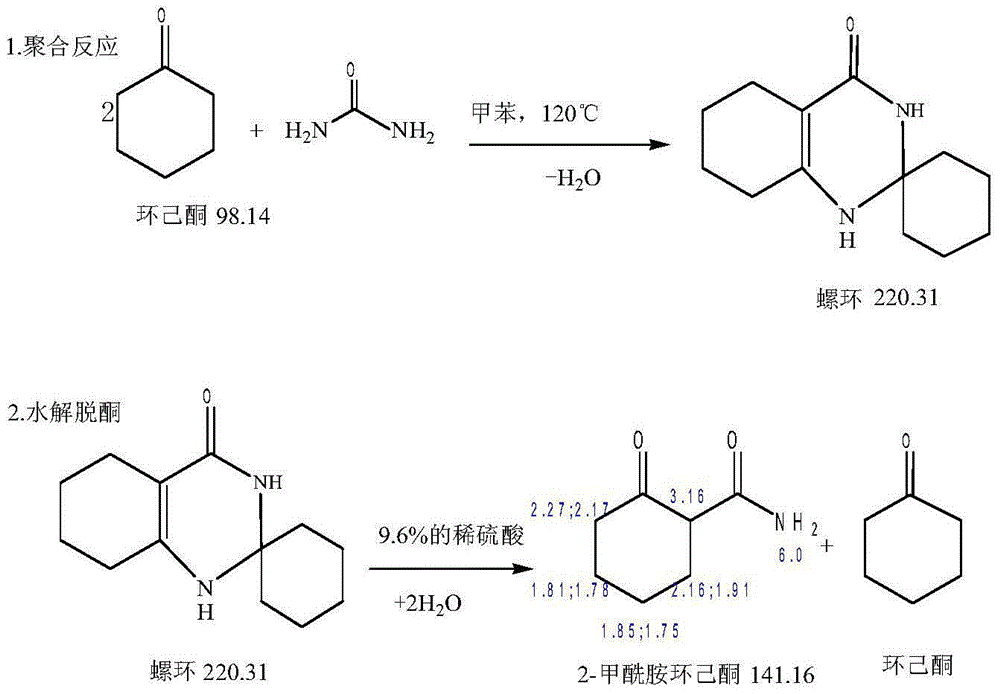
A kind of method of synthesizing 2-carboxamide cyclohexanone
A technology of cyclohexanone and formamide, which is applied in the field of synthesizing 2-formamide cyclohexanone, can solve the problems of low utilization rate of cyclohexanone, pollute the environment and the like, and achieve the advantages of shortened reaction time, low energy consumption and good promotion prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0028] Add 124g of cyclohexanone, 42g of urea, 0.6g of 85.0% phosphoric acid and 15g of xylene into a 1000ml three-neck flask, reflux at 138°C for 2.5 hours, then add 320g of 1M dilute H 2 SO 4 The spiro ring was hydrolyzed by heating. After the reaction was completed, 77 g of the xylene cyclohexanone mixture was collected to obtain 58 g of 2-carboxamide cyclohexanone, mp: 127.1-129.0°C. Based on the consumption of cyclohexanone (62 g), the total yield was 65.3%.
Embodiment 3
[0030] 77g of xylene cyclohexanone mixture collected in Example 2 and 62g of neocyclohexanone, 42g of urea, 0.6g of 85.0% phosphoric acid were added to a 1000ml three-necked flask, and 322g was added after reflux reaction at 135°C for 3 hours to prepare by recovering water from Example 2 1M dilute H2SO4 was heated to hydrolyze the spiro ring. After the reaction was completed, 89 g of the xylene cyclohexanone mixture was collected to obtain 56.2 g of 2-carboxamide cyclohexanone. Yield 78.1%.
Embodiment 4
[0032] Add 124g of cyclohexanone, 42g of urea, 0.6g of 85.0% phosphoric acid and 15g of toluene into a 1000ml three-neck flask, reflux at 136°C for 3 hours, then add 320g of 1M dilute H 2 SO 4 Heated and hydrolyzed the spiro ring. After the reaction was completed, 78.4 g of the tolyl cyclohexanone mixture was collected to obtain 57.8 g of 2-carboxamide cyclohexanone, mp: 127.2-129.5°C. Based on the consumption of 60.6 g of cyclohexanone, the total yield was 66.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com