Ester derivative containing acrylamide indole, preparation method and application thereof
The technology of acrylamido indole and acrylamide group is applied in the field of ester derivatives containing acrylamido indole and the preparation thereof, and can solve the problems of reduced antifouling period effect, fast release rate, poor compounding performance and the like , to achieve the effect of uniform release, preventing adhesion and good antifouling performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
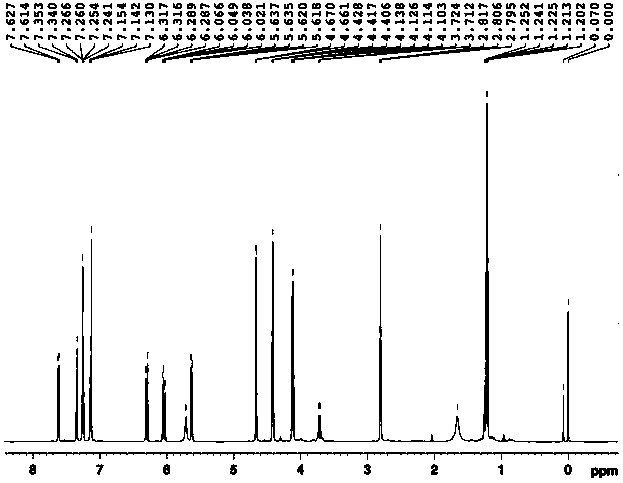
[0047] Embodiment 1: Preparation of 3-(3-((acrylamido) methyl)-1-indolyl) propionic acid methyl ester
[0048] 1. Preparation of 3-(1-indolyl) methyl propionate
[0049] Weigh 5.8g (0.05mol) of indole and 11.9g (0.05mol) of dimethyl β-dithiodipropionate and dissolve them in 50mL of anhydrous tetrahydrofuran (THF). Add 50mL of anhydrous THF and 3.6g (0.09mol) NaH (60% content) in sequence into a 250mL three-necked flask equipped with a stirring device and a thermometer, and add the THF solution of indole to the above system dropwise at 0°C. After dropping, the reaction was continued for 30 minutes, and the THF solution of dimethyl β-dithiodipropionate was added dropwise to the above system while maintaining 0°C. After dropping, the reaction was continued at room temperature for 3 hours. After the reaction, add 50mL of saturated NH 4 Cl solution, let it stand, take the oil layer, evaporate the solvent, wash with water, MgSO 4 A reddish-brown oil was obtained after drying for...
Embodiment 2
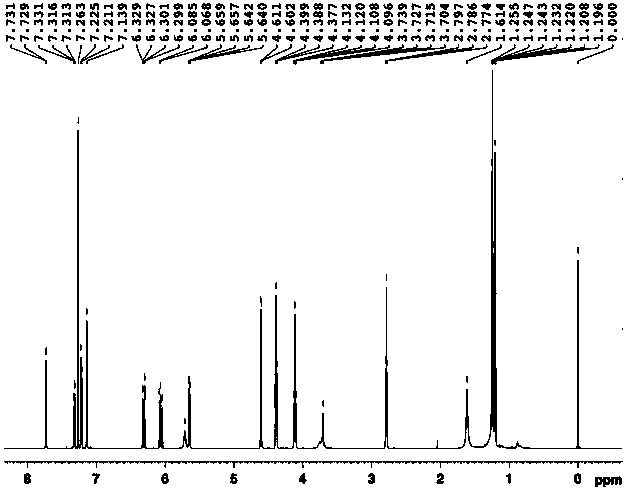
[0054] Example 2: Preparation of 3-(3-((acrylamido)methyl)-5-bromo-1-indolyl)propionic acid methyl ester
[0055] 1. Preparation of methyl 3-(5-bromo-1-indolyl)propionate
[0056] Weigh 9.8g (0.05mol) of 5-bromoindole and 11.9g (0.05mol) of dimethyl β-dithiodipropionate and dissolve them in 50mL of anhydrous tetrahydrofuran (THF). Add 50mL of anhydrous THF and 3.6g (0.09mol) NaH (60% content) in sequence into a 250mL three-necked flask equipped with a stirring device and a thermometer, and add the THF solution of indole to the above system dropwise at 0°C. After dropping, the reaction was continued for 30 minutes, and the THF solution of dimethyl β-dithiodipropionate was added dropwise to the above system while maintaining 0°C. After dropping, the reaction was continued at room temperature for 3 hours. After the reaction, add 50mL of saturated NH 4 Cl solution, let it stand, take the oil layer, evaporate the solvent, wash with water, MgSO 4 A reddish-brown oil was obtained...
Embodiment 3
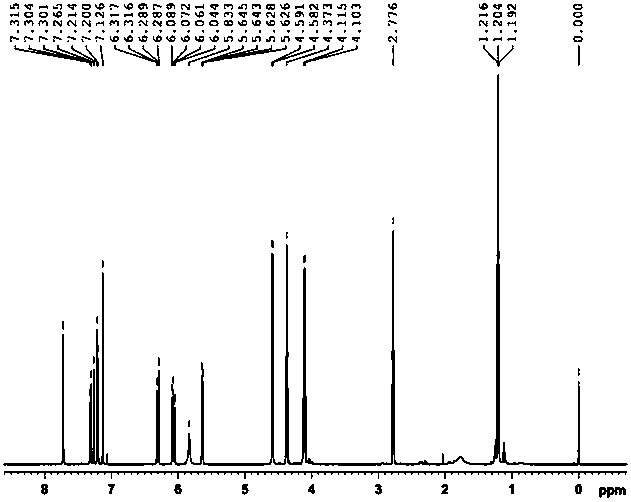
[0061] Example 3: Preparation of ethyl 3-(3-((acrylamido)methyl)-5-bromo-1-indolyl)propionate
[0062] 1. Preparation of ethyl 3-(5-bromo-1-indolyl)propionate
[0063] Weigh 9.8g (0.05mol) of 5-bromoindole and 13.3g (0.05mol) of diethyl β-dithiodipropionate and dissolve them in 50mL of anhydrous tetrahydrofuran (THF). Add 50mL of anhydrous THF and 3.6g (0.09mol) NaH (60% content) in sequence into a 250mL three-necked flask equipped with a stirring device and a thermometer, and add the THF solution of indole to the above system dropwise at 0°C. After dropping, continue to react for 30 minutes, then add the THF solution of diethyl β-dithiodipropionate dropwise into the above system while keeping 0°C. After dropping, the reaction was continued at room temperature for 3 hours. After the reaction, add 50mL of saturated NH 4 Cl solution, let it stand, take the oil layer, evaporate the solvent, wash with water, MgSO 4 A reddish-brown oil was obtained after drying for 12 hours. U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com