Drotaverine hydrochloride crystal form I and crystal form II and preparation method
A technology of drotaverine hydrochloride and crystal form, which is applied in the field of preparation of drotaverine hydrochloride, can solve problems such as unfavorable and stable storage, and achieve the effects of good purity and stability, good stability, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of drataverine hydrochloride crystal form I
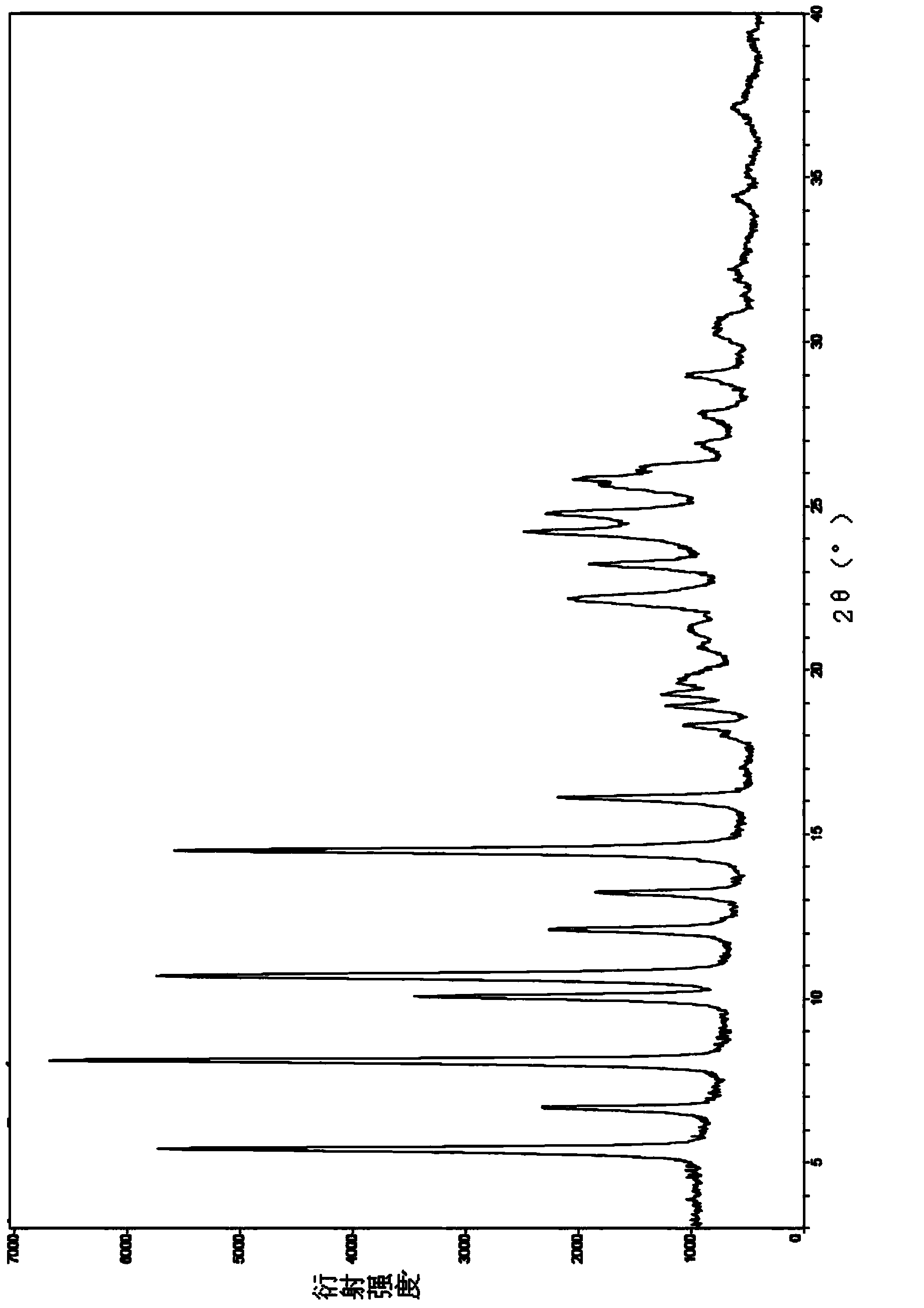
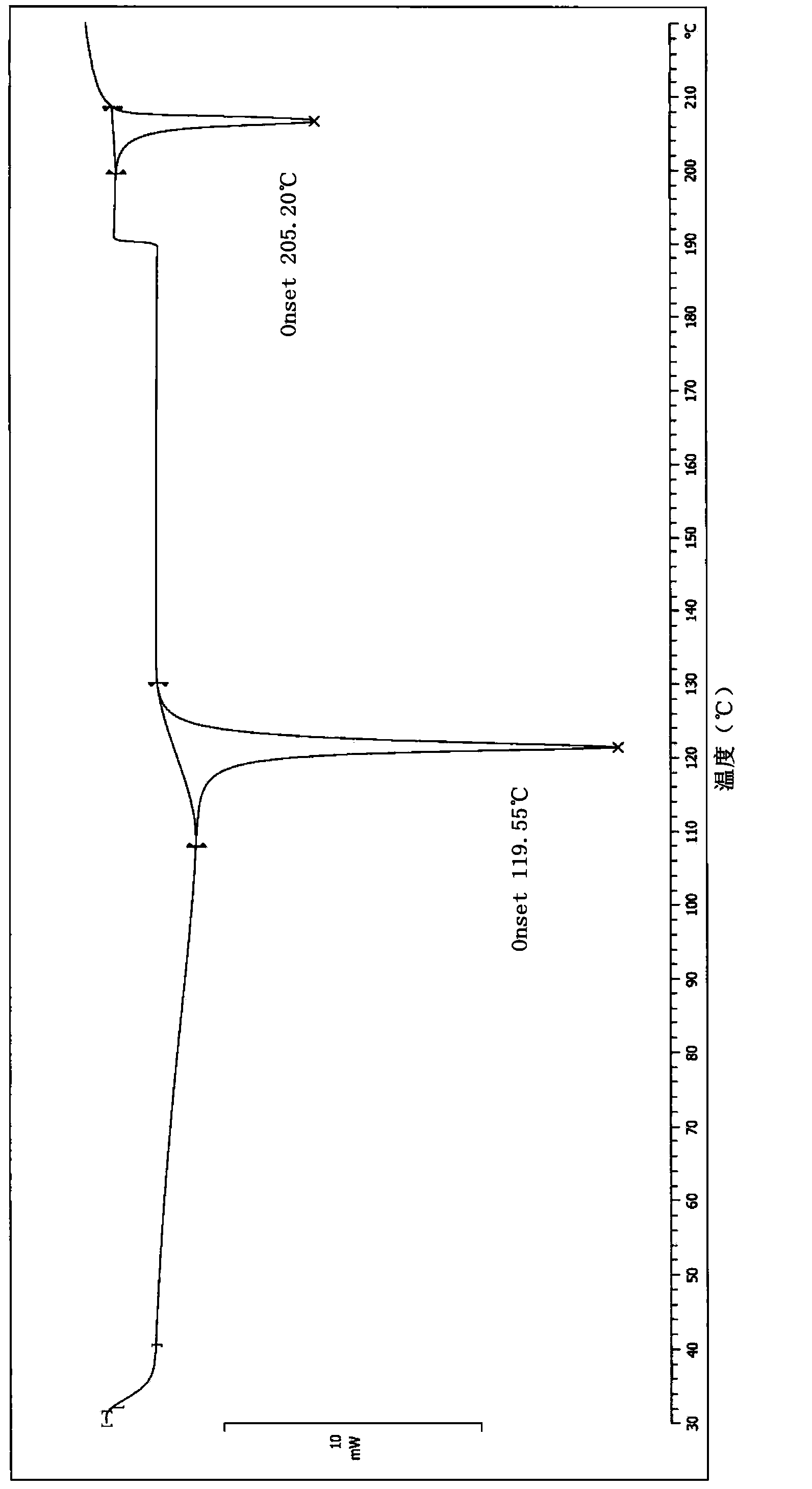
[0046] Put 150mL of purified water, 22.5g of drotaverine hydrochloride, and 4.5mL of concentrated hydrochloric acid into a 250mL three-necked flask, and raise the temperature to 70°C. Most of the system will dissolve, and the undissolved part will turn pale yellow. After stirring for 30 minutes, remove the hot water bath, cool down to 30°C naturally, and filter with suction. The wet product was baked under reduced pressure in an oven at 50°C for 24 hours to obtain Form I with a yield of 86.4% and a water content of 4.0%. The HPLC purity is above 99.5%.
[0047] The H-NMR data of product are as follows:
[0048] 1H-NMR (500MHz, DMSO-d6, TMS) δ13.89 (s, 1H), δ7.59 (s, 1H), δ7.22 (d, J=1.5, 1H), δ7.10 (s, 1H ), δ6.92 (dd, J=8.3, 1.7, 1H), δ6.87 (d, J=8.3, 1H), δ4.53 (s, 2H), δ4.14~4.18 (q, J=6.9 , 2H), δ4.06~4.10 (q, J=6.9, 2H), δ3.98~4.02 (q, J=6.9, 2H), δ3.93~3.97 (q, J=6.8, 2H), δ3 .82 (t, J=7.9, 2H), δ2.97 ...
Embodiment 2
[0056] Preparation of drataverine hydrochloride crystal form II
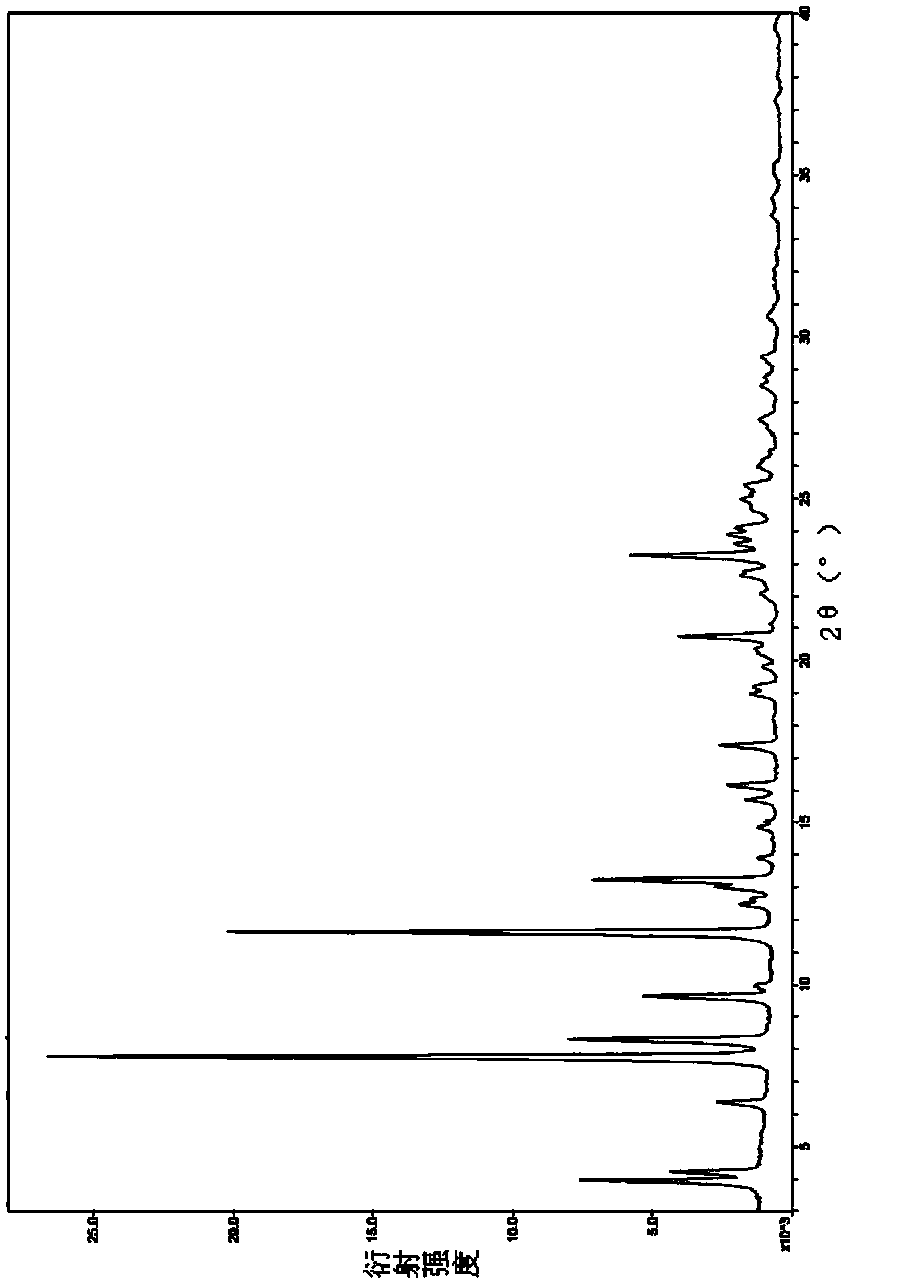
[0057] Preparation of crystal form II of drotaverine hydrochloride: 150 mL of purified water, 22.5 g of drotaverine hydrochloride, and 4.5 mL of concentrated hydrochloric acid were put into a 250 mL three-necked flask, and the temperature was raised to 80°C. After the system was dissolved, cool it in a tap water bath, lower the temperature to 31°C, filter with suction, and dry the wet product in a 40°C oven under reduced pressure for 24 hours to obtain Form II, with a yield of 88.1% and a moisture content of 5.6%. HPLC purity is more than 99.5%, and nuclear magnetic detection data is with embodiment 1.
[0058] The sample prepared by this embodiment is subjected to X-ray powder diffraction detection (the detection instrument and scanning conditions are the same as in Example 1), and the detection results are shown in image 3 , image 3 The main characteristic peak parameters in are shown in the following tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com