Iridium complex organic fluorescent nanoparticles and preparation method thereof
An iridium complex, fluorescent nanotechnology, applied in nanotechnology, organic chemistry, nanotechnology and other directions, can solve the problem of less research on phosphorescent heavy metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of dichloro bridge (dfppy) 2 Ir(μ-Cl) 2 (dfppy) 2 : Under nitrogen protection, take IrCl with a molar ratio of 2:5 3 ·3H 2 O and dfppy, add a mixed solvent of 2-ethoxyethanol and water with a volume ratio of 3:1, heat the above mixed solution to 110° C., stir for 24 hours, cool, filter, and wash.
[0025] (2) preparation (dfppy) 2 Ir(μ-Cl) 2 (dfppy) 2 PEO-b-P4VP: dichloro bridge of iridium complex (dfppy) 2 Ir(μ-Cl) 2 (dfppy) 2 The molar ratio to PEO-b-P4VP is 12:1; the concentration of the dichloro bridge of the iridium complex in DMF is 4.5mmol / L; after stirring overnight, dialyze with deionized water for one day.
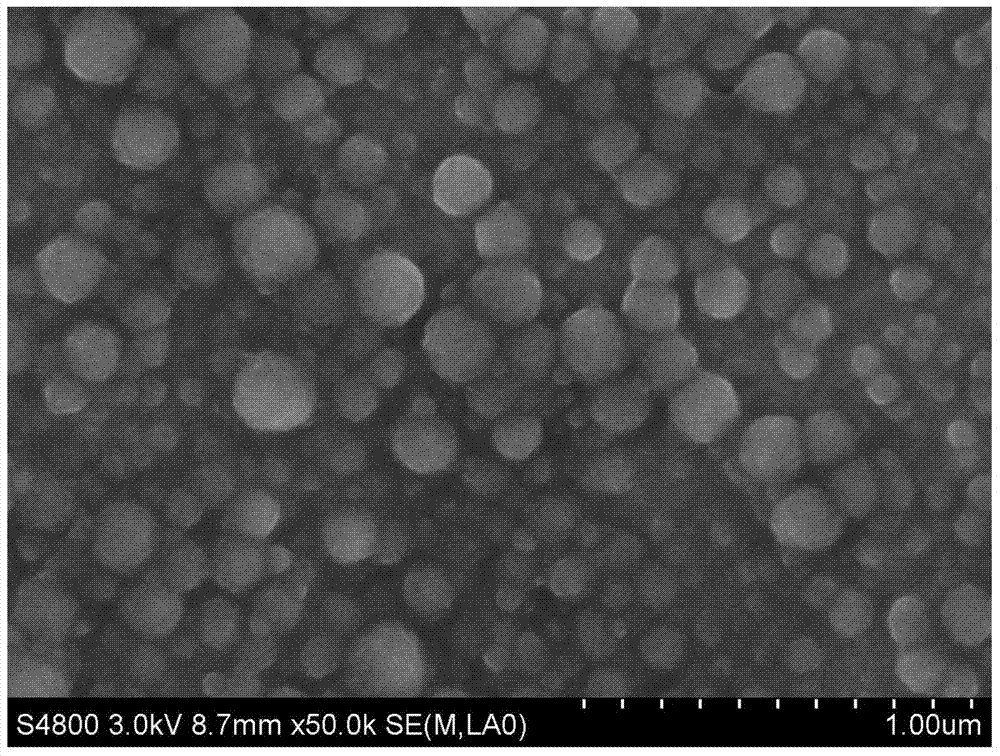
[0026] The results of this example are as figure 1 As shown by the FESEM electron microscope, all of them are spherical in shape with a size of 50-100nm.
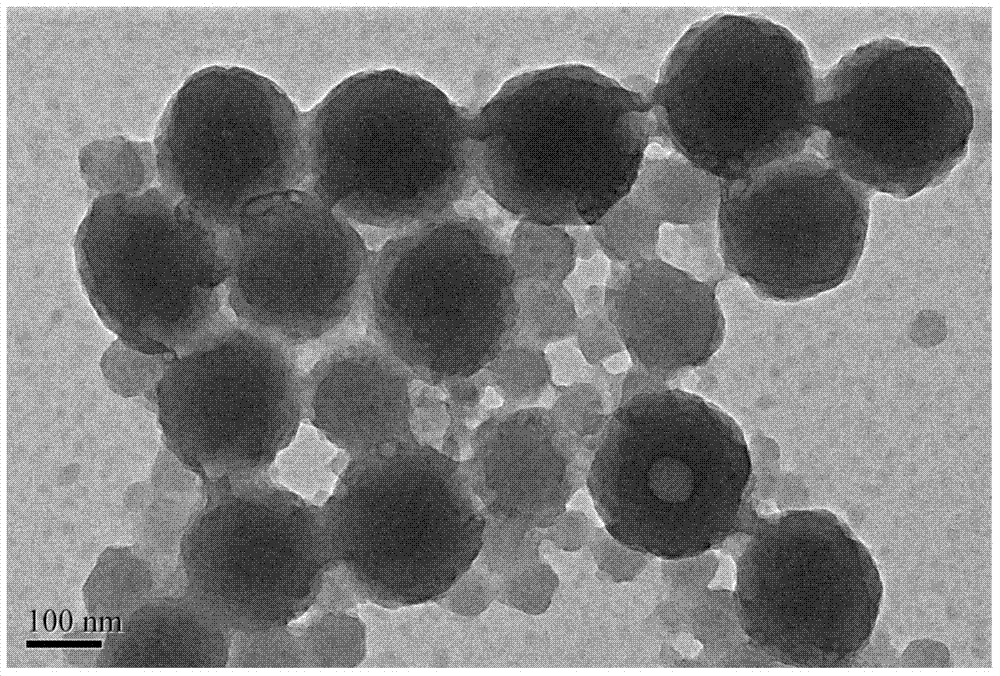
[0027] figure 2 It is the TEM electron microscope result of this embodiment.
Embodiment 2
[0029] (1) Preparation of dichloro bridge (ppy) 2 Ir(μ-Cl) 2 (ppy) 2 : Under nitrogen protection, take IrCl with a molar ratio of 1:3 3 ·3H 2 O and polypyrrole, add a mixed solvent of 2-ethoxyethanol and water with a volume ratio of 3:1, heat the above mixed solution to 110°C, stir for 24 hours, cool, filter, and wash.
[0030] (2) preparation (ppy) 2 Ir(μ-Cl) 2 (ppy) 2 PEO-b-P4VP:
[0031] Dichloro Bridge of Iridium Complexes (ppy) 2 Ir(μ-Cl) 2 (ppy) 2 The molar ratio to PEO-b-P4VP is 12:1; the concentration of the dichloro bridge of the iridium complex in DMF is 4.5mmol / L; after stirring overnight, dialyze with deionized water for one day.
[0032] The FESEM electron microscope figure of the product obtained in this embodiment is as follows image 3 As shown, most of them are spherical in shape with a size of 50-100nm.
[0033] Figure 4 It is the TEM electron microscope result of this embodiment.
Embodiment 3
[0035] (1) Preparation of dichloro bridge (pq) 2 Ir(μ-Cl) 2 (pq) 2 : Under nitrogen protection, take IrCl with a molar ratio of 1:3 3 ·3H 2 O and pq, add a mixed solvent of 2-ethoxyethanol and water with a volume ratio of 3:1, heat the above mixed solution to 110°C, stir for 24 hours, cool, filter, and wash.
[0036] (2) Preparation (pq) 2 Ir(μ-Cl) 2 (pq) 2 PEO-b-P4VP: dichloro bridge of iridium complex (pq) 2 Ir(μ-Cl) 2 (pq) 2 The molar ratio to PEO-b-P4VP is 12:1; the concentration of the dichloro bridge of the iridium complex in DMSO is 4.5mmol / L; after stirring overnight, dialyze with deionized water for one day.
[0037] The FESEM electron microscope figure of the product obtained in this embodiment is as follows Figure 5 As shown, most of them are spherical in shape with a size of 100-150 nm.
[0038] Figure 6 It is the TEM electron microscope result of this embodiment.
[0039] The fluorescence spectrum of the iridium complex organic fluorescent nanoparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com