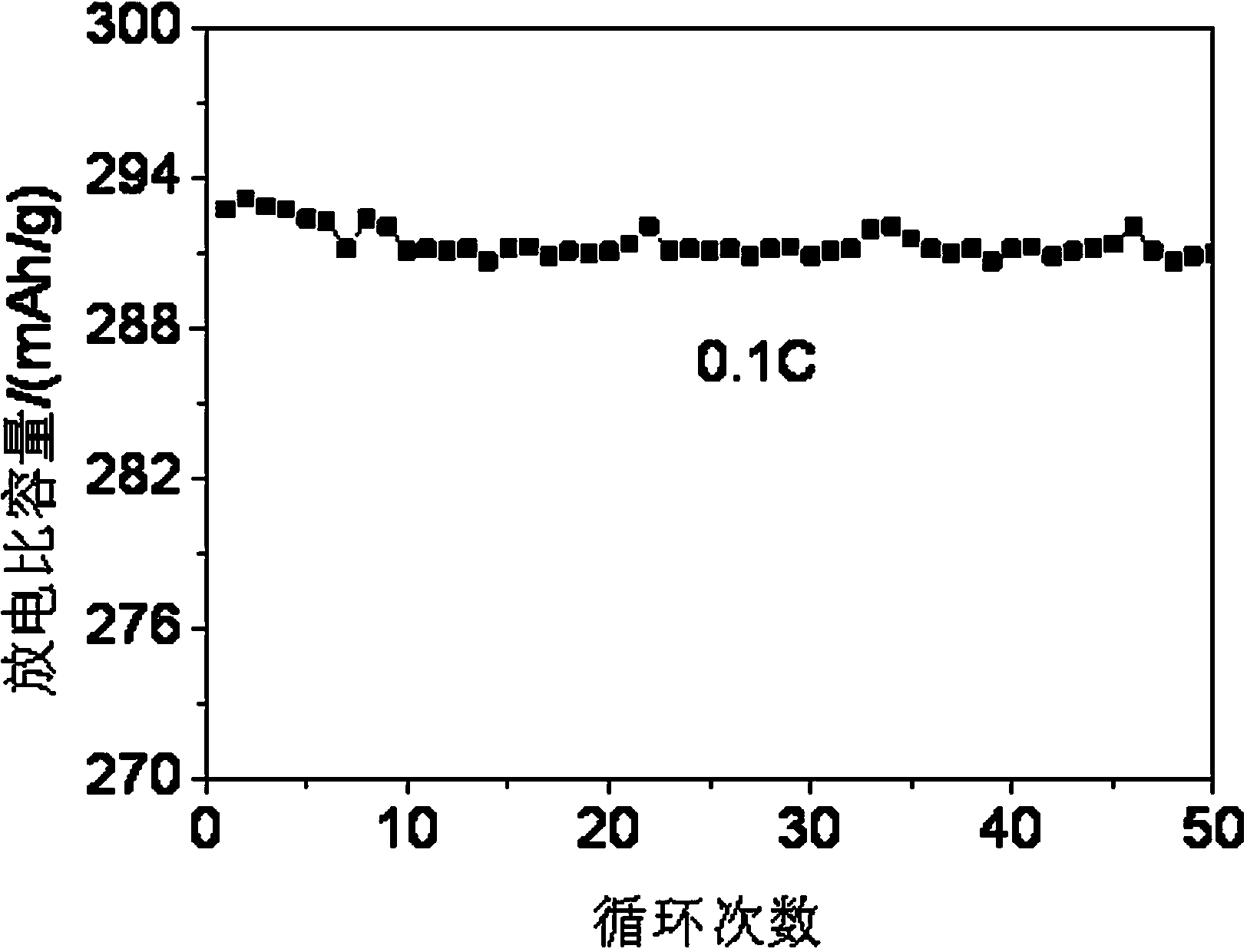
Coated spherical Li-rich cathode material and preparation method thereof
A lithium-rich cathode material and coating technology, which is applied in the field of lithium-ion battery cathode materials and its preparation, can solve the problems of destroying the surface lattice structure of materials, poor material rate performance, poor compatibility, etc., and achieve simple and time-saving operation , short diffusion path and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of carbon-coated spherical lithium-rich materials Li 1.2 mn 0.54 Ni 0.13 co 0.13 o 2 According to the stoichiometric ratio of Li, Ni, Co, Mn in the target product, accurately weigh the corresponding nitrate, use citric acid as complexing agent, according to the total molar weight of metal salt: molar weight of citric acid=1:1, Prepare a citric acid solution, add it into the metal nitrate mixture at a certain speed, and adjust the pH to 5; keep the mixture at a constant temperature of 80 o C stirring, after sol, gel, and curing, put the cured material sample in the air atmosphere of the tube furnace at 550 o C pre-decomposition 3h, then 800 o C roasting for 12h, grinding and sieving to obtain Li with a particle size of 30-40nm 1.2 mn 0.54 Ni 0.13 co 0.13 o 2 particles.
[0029] Li 1.2 mn 0.54 Ni 0.13 co 0.13 o 2 Nanoparticles and glucose were mixed at a mass ratio of 1:0.5 to form a uniformly dispersed suspension with a solid content of about ...
Embodiment 2
[0032] Preparation of carbon-coated spherical lithium-rich materials Li 1.2 mn 0.56 Ni 0.13 co 0.13 o 2 According to the stoichiometric ratio of each element of Li, Ni, Co, Mn in the target product, accurately weigh the corresponding acetate, use polyacrylic acid as complexing agent, according to the total molar weight of metal salt: polyacrylic acid molar weight=1:1.3 , prepare polyacrylic acid solution, add metal acetate mixed solution at a certain speed, adjust pH to 2; keep mixed solution constant temperature 80 o C stirring, after sol, gel, and curing, put the cured material sample in the air atmosphere of the tube furnace at 400 o C pre-decomposition 3h, then 800 o C roasting for 12h, grinding and sieving to obtain Li with a particle size of 20-30nm 0.2 mn 0.54 Ni 0.13 co 0.13 particles.
[0033] Li 0.2 mn 0.54 Ni 0.13 co 0.13 Nanoparticles and polyvinylpyrrolidone were mixed at a mass ratio of 1:0.05 to form a uniformly dispersed suspension with a solid co...
Embodiment 3
[0035] Preparation of carbon-coated spherical lithium-rich materials Li 1.2 mn 0.56 Ni 0.13 co 0.13 o 2 Accurately weigh any proportion of acetate and nitrate according to the stoichiometric ratio of Li, Ni, Co, Mn metal elements in the target product, use polyacrylic acid as complexing agent, according to the total molar weight of metal salt: polyacrylic acid mole Quantity = 1:1.3, make polyacrylic acid solution, add metal acetate mixture at a certain speed, adjust pH to 2; keep the temperature of the mixture at 80 o C stirring, after sol, gel, and curing, put the cured material sample in the air atmosphere of the tube furnace at 550 o C pre-decomposition 3h, then 800 o C roasting for 12h, grinding and sieving to obtain Li with a particle size of 20-30nm 0.2 mn 0.54 Ni 0.13 co 0.13 particles.
[0036] Li 0.2 mn 0.54 Ni 0.13 co 0.13 Nanoparticles, glucose and polyvinylpyrrolidone were mixed according to the mass ratio of 1:0.4:0.02 to form a uniformly dispersed s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com