Meloxicam injection for treating rheumatoid arthritis and osteoarthritis and preparation method thereof
A technology for osteoarthritis and meloxicam, applied in anti-inflammatory agents, bone diseases, pharmaceutical formulations, etc., can solve the problems of slow absorption and low bioavailability, achieve fast absorption, good clinical application value, and ease Effects of pain symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
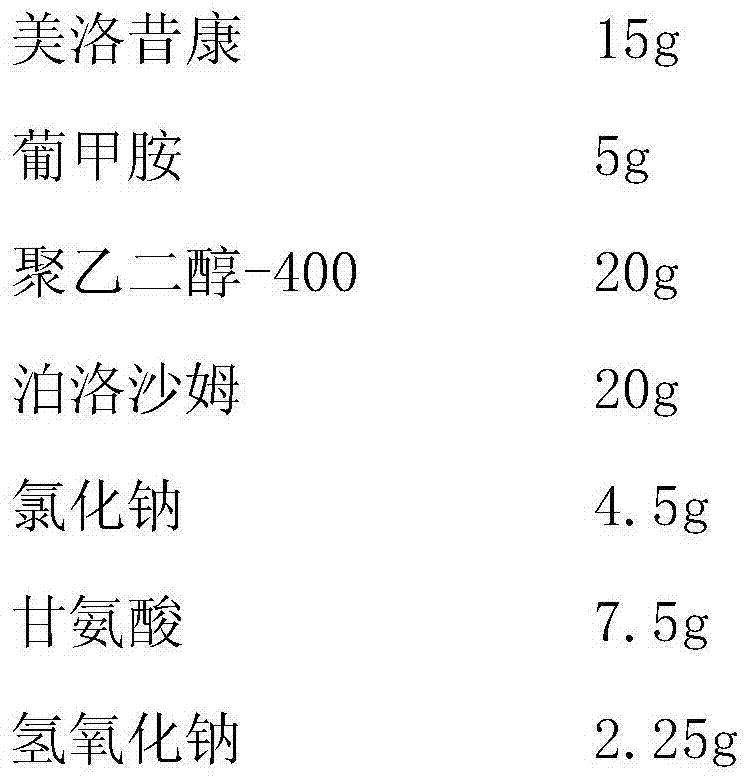
[0022] (1) Weighing
[0023]
[0024] (2) Roughly wash, finely wash, and dry the selected ampoule after passing the inspection;
[0025] (3) Add 1000ml of water for injection into the batching tank, put the taken meglumine, polyethylene glycol-400, poloxamer, sodium chloride, glycine and sodium hydroxide into the batching tank, stir to dissolve , add meloxicam, heat up to about 80°C under stirring to dissolve, and control the pH value to 8.5-10.0 by adding or subtracting the amount of sodium hydroxide;
[0026] (4) Add 0.05g of activated carbon for needles, stir and absorb for 20-30 minutes, remove the carbon by coarse filtration, and add water for injection to 1500ml;
[0027] (5) Filter through a 0.45 μm microporous membrane until the solution is clear, potting 1,000 tubes, and then sterilize with 100°C circulating steam for 30 minutes.
Embodiment 2
[0029] (1) Weighing
[0030]
[0031]
[0032] (2) Roughly wash, finely wash, and dry the selected ampoule after passing the inspection;
[0033] (3) Add 1000ml of water for injection into the batching tank, put meglumine, polyethylene glycol-400, poloxamer, sodium chloride, glycine and sodium hydroxide into the batching tank, stir to dissolve, and then Add meloxicam, heat up to about 80°C under stirring to dissolve, and control the pH value to 8.5-10.0 by adding or subtracting the amount of sodium hydroxide;
[0034] (4) Add 0.05% (g / ml) activated carbon for needles, stir and adsorb for 20-30 minutes, filter to remove carbon, add water for injection to 1500ml;
[0035] (5) Filter through a 0.45 μm microporous membrane until the solution is clear, potting 1,000 tubes, and then sterilize with 100°C circulating steam for 30 minutes.
Embodiment 3
[0037] (1) Weighing:
[0038]
[0039] (2) Roughly wash, finely wash, and dry the selected ampoule after passing the inspection;
[0040] (3) Add 1000ml of water for injection into the batching tank, weigh the prescribed amount of meglumine, polyethylene glycol-400, poloxamer, sodium chloride, glycine and sodium hydroxide, put them into the batching tank, and stir Make it dissolve, add the prescribed amount of meloxicam, heat up to about 80°C under stirring to dissolve, and control the pH value from 8.5 to 10.0 by adding or subtracting the amount of sodium hydroxide;
[0041] (4) Add 0.05g of activated carbon for needles, stir and absorb for 20-30 minutes, remove the carbon by coarse filtration, and add water for injection to 1500ml;
[0042] (5) Filter through a 0.45 μm microporous membrane until the solution is clear, potting 1,000 tubes, and then sterilize with 100°C circulating steam for 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com