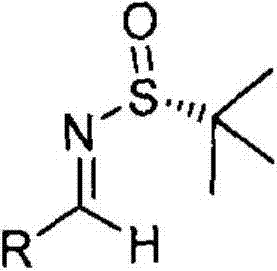
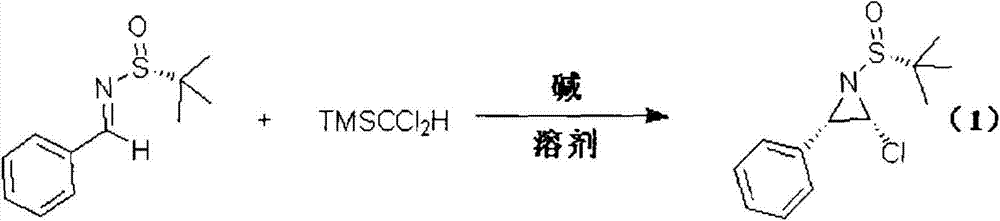
Method for preparing chiral alpha-chloroaziridine
A technology of aziridine and chirality, which is applied in the field of preparation of chiral α-chloroaziridines, can solve the problem of high-efficiency chiral synthesis methods of α-chloroaziridines that have not been reported and are not widely used. problems such as adaptability and selectivity, to achieve the effect of mild process conditions and economical and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
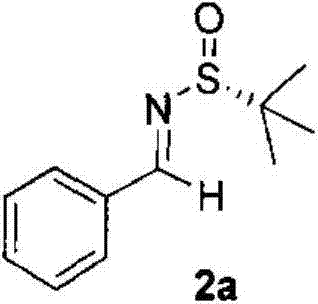
[0024] At -70°C, sodium tert-butoxide (0.96 g, 10 mmol, dissolved in 1.0 ml tetrahydrofuran) was slowly added dropwise into 2 H) (1.57 g, 10 mmol), imine represented by formula (2a) (2.1 g, 10 mmol), and 15 ml of tetrahydrofuran in a reaction flask. After reacting for 1 hour, 10 ml of water was added to terminate the reaction. The reaction solution was then transferred to a separatory funnel and extracted with ethyl acetate (50ml×2). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Flash column chromatography with ethyl acetate / petroleum ether (volume ratio 1:5) yielded 1.62 g of product 3a with a yield of 62% and a dr of 99:1.
[0025]
[0026] Characterization data of compound 3a:
[0027]
[0028] Pale yellow liquid; mp71.1-71.8℃; [α] D 25 +54.4(c0.51, CHCl 3 ); IR (film) 2917, 1450, 1285, 1078, 918, 699cm -1 ; 1 H NMR (CDCl 3 )δ7.39(ddd, J=7.3, 4.2, 1.5Hz, 5H), 4.57(d, J=5.6Hz, 1H), 3.93(d, J=5....
Embodiment 2
[0030] At a temperature of -20°C, sodium tert-butoxide (1.92 g, 20 mmol, dissolved in 1.0 ml of N, N-dimethylformamide) was slowly added dropwise into a solution containing trimethyl(dichloromethyl)silane (TMSCCl 2 H) (3.14 g, 20 mmol), imine represented by formula (2b) (2.23 g, 10 mmol), and 15 ml of N,N-dimethylformamide in a reaction flask. After reacting for 3 hours, 10 ml of water was added to terminate the reaction. The reaction solution was then transferred to a separatory funnel and extracted with ethyl acetate (50ml×2). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Flash column chromatography with ethyl acetate / petroleum ether (volume ratio 1:4) yielded 2.30 g of product 3b with a yield of 85% and a dr of 98:2.
[0031]
[0032] Characterization data of compound 3b:
[0033]
[0034] Pale yellow liquid; [α] D 25 +69.6 (c0.59, CHCl 3 ); IR (film) 2924, 1459, 1290, 1086, 927, 707cm -1 ; 1 H...
Embodiment 3
[0036]At a temperature of 0°C, potassium tert-butoxide (2.8 g, 25 mmol, dissolved in 2.0 ml dimethyl sulfoxide) was slowly added dropwise into 2 H) (3.92 g, 25 mmol), imine represented by formula (2c) (2.23 g, 10 mmol), and 15 ml of dimethyl sulfoxide in a reaction flask. After reacting for 3 hours, 10 ml of water was added to terminate the reaction. The reaction solution was then transferred to a separatory funnel and extracted with ethyl acetate (50ml×2). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Flash column chromatography with ethyl acetate / petroleum ether (1:4) gave 2.44 g of product 3b with a yield of 90% and a dr of 99:1.
[0037]
[0038] Characterization data of compound 3c:
[0039]
[0040] Colorless liquid; [α] D 25 +155.9 (c0.95, CHCl 3 ); IR (film) 2923, 1477, 1293, 1082, 948, 810, 698cm -1 ; 1 H NMR (CDCl 3 )δ7.29-7.15 (m, 4H), 4.55 (d, J = 5.6Hz, 1H), 3.90 (d, J = 5.6Hz, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com