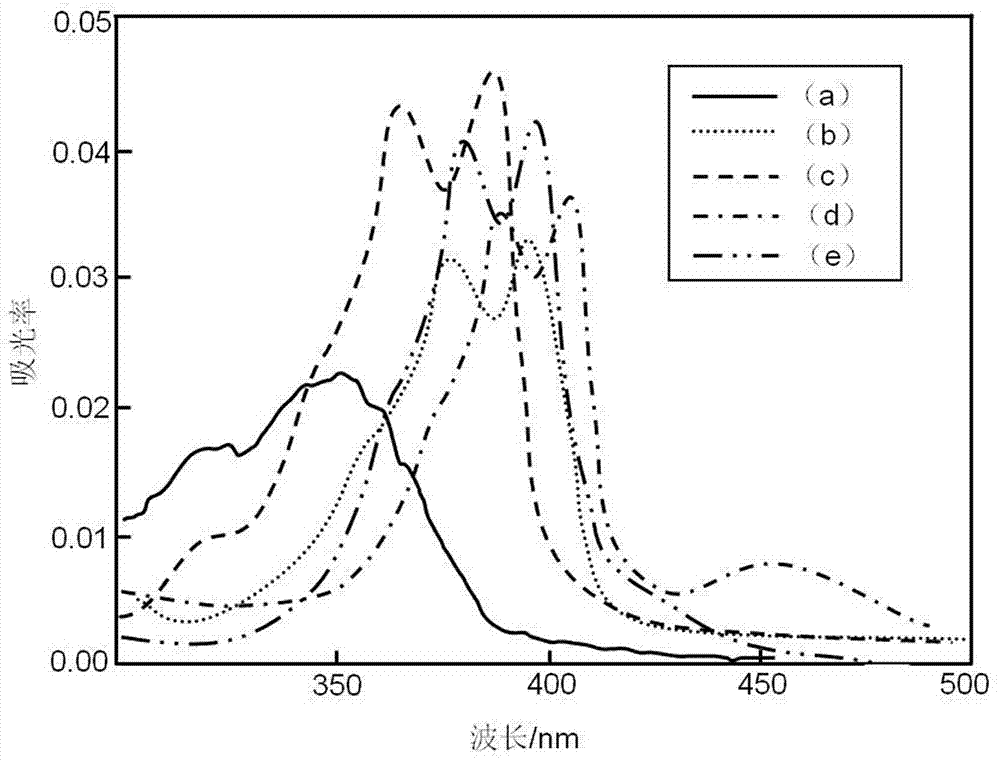
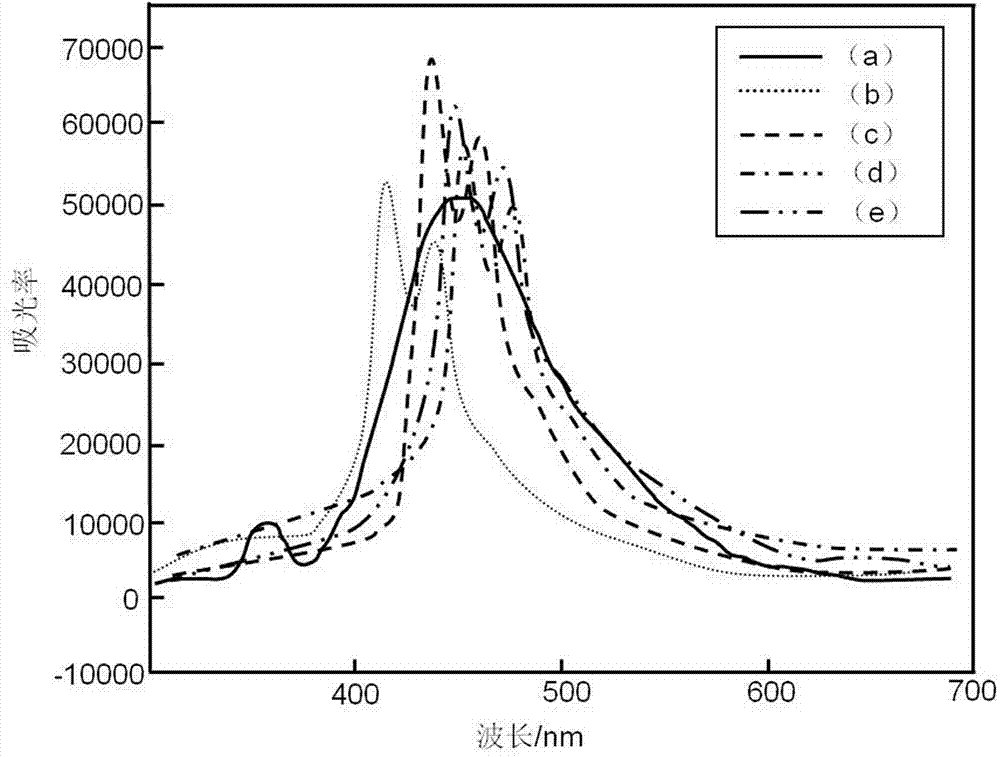
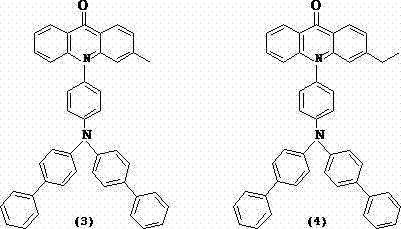
Acridone derivatives and synthesis method thereof
A derivative, acridone technology, applied in the field of organic chemical synthesis, can solve the problem of large fluorescence influence, and achieve the effect of good fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthetic compound (1)
[0038] ① Add 160g dimethyl sulfoxide into a reaction flask with a thermometer and a reflux tube, and add (b) 156g (1mol), 70g potassium carbonate, CuFe while stirring. 2 O 4 Magnetic powder 6g and (a) 94g (1mol), react at 100~105℃, TLC tracking shows that the raw material has reacted completely, add cooling water to cool the system to 60~80℃, add 2g activated carbon and 2g sodium sulfide nonahydrate, continue stirring until After the system is uniform, filter, then add concentrated hydrochloric acid to adjust the pH of the filtrate between 1 and 2, filter the precipitate, wash with water, and dry to obtain 190g of white solid powder intermediate (c);
[0039] ② Add 1000g of toluene, 100g (0.47mol) of intermediate (c) and 80g of p-toluenesulfonic acid into the reaction flask, and react at 100~110℃. TLC tracking shows that the reaction of the raw materials is completed and the temperature is reduced to 20~25℃ to precipitate the result. 80g of yellow-gr...
Embodiment 2
[0042] The compound (2) was synthesized using the same method, with a purity of 99.3% and a yield of 76%, MS: [M+1]: 605; 1 HNMR (DMSO- d 6 )Δ: 7.80 (d, 1H), 7.62 (s, 1H), 7.22 (m, 2H), 7.46 (d, 4H), 7.21 (m, 10H), 6.80 (m, 1H), 6.62 (m, 2H) ),6.53 (d,4H),6.20 (d,4H), 2.30(s,3H); Anal. Calcd for C 44 H 32 N 2 O: C 87.39, H 5.33, N 4.63; found C 87.40, H 5.33, N 4.60.
Embodiment 3
[0044] Compound (3) was synthesized using the same method, with a purity of 99.5% and a yield of 72%, MS: [M+1]: 605; 1 HNMR (DMSO- d 6 )Δ: 7.81 (d,1H), 7.66 (s,1H), 7.45 (d,4H), 7.32 (m,1H), 7.21 (m,10H), 6.70 (m,3H), 6.55 (d,4H) ), 6.30(s,1H), 6.21 (d,4H), 2.35(s,3H); Anal. Calcd for C 44 H 32 N 2 O: C 87.39, H 5.33, N 4.63; found C 87.39, H 5.34, N 4.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com