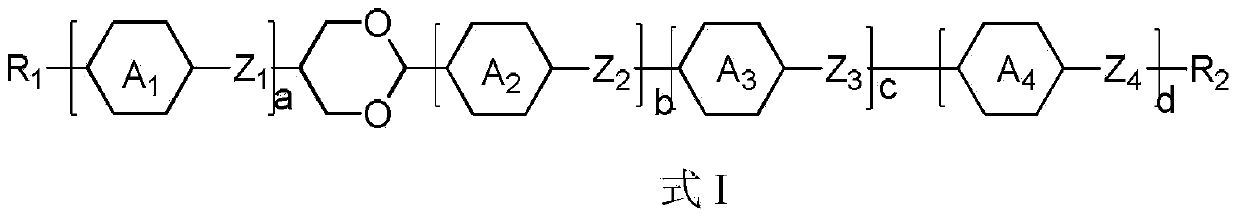
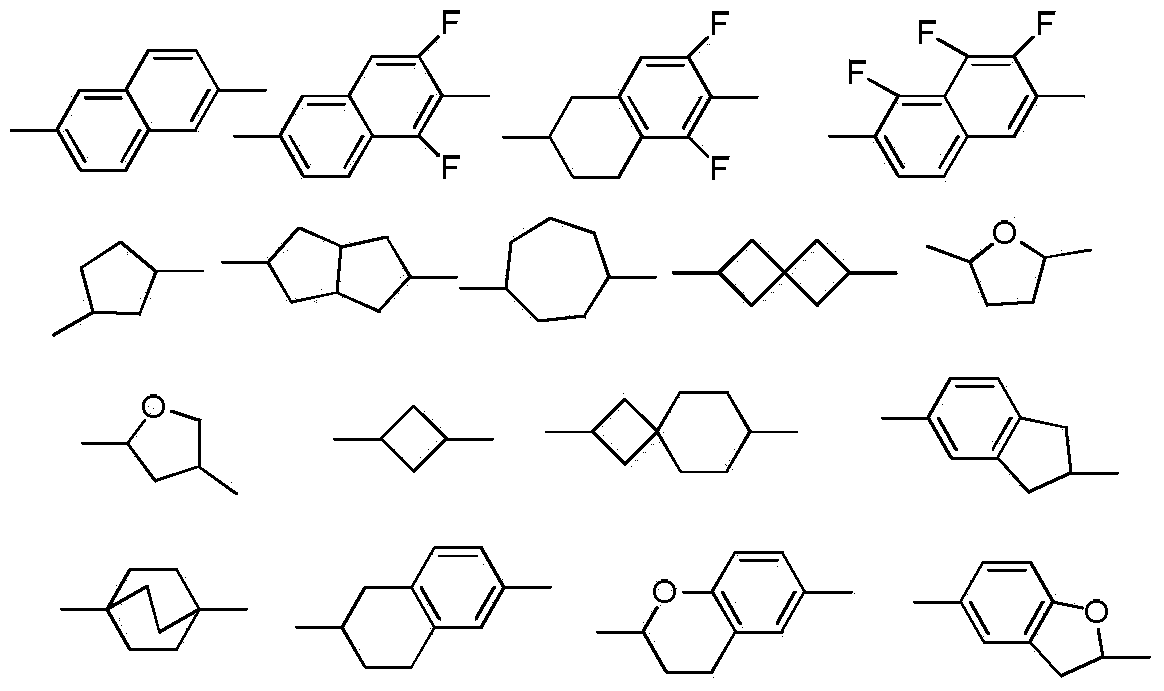
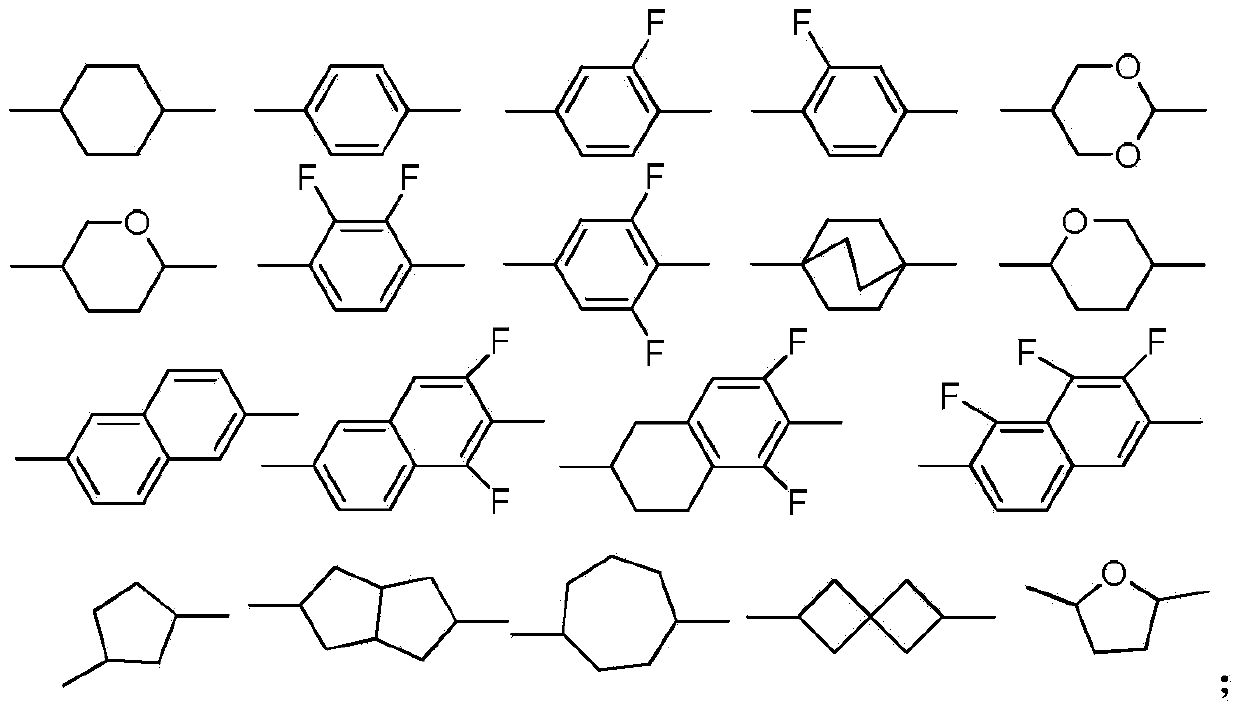
Dioxane derivatives as well as preparation method and application thereof
A kind of technology of alkyl and compound, applied in the field of dioxane derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1, Synthesis of (method 1)
[0111]
[0112] Step 1: Synthesis of I-17-a
[0113] Add 68g (1mol) sodium ethoxide and 1L absolute ethanol to the reaction flask, add 132g (1mol) dimethyl malonate under stirring and heat to reflux for 1 hour, 149g (1mol) cyclopentyl bromide (reactant) is added dropwise to the reaction flask In the bottle, reflux and stir for 2 hours, add 1L of water, 1L of n-heptane, process according to the conventional method, combine the organic phase and spin dry the solvent, and distill under reduced pressure to obtain 160g of the product, with a yield of 80%.
[0114] Step 2: Synthesis of I-17-b
[0115] Add 38g of lithium aluminum hydride and 1L of tetrahydrofuran to a 2L three-necked flask, add 160g (0.8mol) of I-17-a (reactant) dropwise under stirring, heat and stir under reflux for 8 hours, cool down to 0°C, and drop in 105g of potassium sodium tartrate The solution prepared with 300g of water was left to stand for 10 minutes, a...
Embodiment 2
[0139] Embodiment 2, compound Synthesis of (method 2)
[0140]
[0141] Add 30.4g (0.11mol) (I-17-d) (reactant) to the reaction flask, 38.9g (0.1mol) (Reactant) (synthesized according to Peer.Kirsch et al., Angew.Chem.Int.Ed.2001.40.1480.), tetrakis (triphenylphosphine) palladium 0.3g (catalyst), sodium carbonate 15g (catalyst), toluene 100ml (solvent), 100ml water, 100ml ethanol (solvent), heated to reflux for 4 hours, added 100ml water, separated, the organic phase was evaporated to dryness, and recrystallized by column chromatography to obtain 37.8g of the product with a yield of 70%.
[0142] The structural confirmation data of this product are as follows:
[0143] GC: 99.9%;
[0144] MS: m / s%540 (M + 1.2) 393 (100) 267 (82.3) 239 (57.8)
[0145] 1H-NMR: δ (ppm)
[0146] 1.35(m,2H)1.56(m,8H)3.64(m,2H)3.88(m,2H)5.98(s,1H)6.89(m,2H)7.22(d,2H)7.40(m,4H)
[0147] As can be seen from the above, the product has a correct structure and is a compound shown in formula I...
Embodiment 3
[0154] Embodiment 3, Synthesis of (Method 3)
[0155]
[0156] Step 1: Synthesis of I-19-a
[0157] In a 500ml three-necked flask, add 0.1mol I-17-b and 12.4g (0.1mol) of 3-fluorobenzaldehyde (reactant) in a solution of 250ml toluene (solvent), then add 2g p-toluenesulfonic acid, The mixture was stirred and refluxed for 3 hours, cooled naturally to 20°C, added 50ml of water, separated, the organic phase was washed with 4×50ml of water, and the solvent was evaporated to dryness to obtain I-19-a
[0158] Step 2: Synthesis of I-19-b
[0159] Add 40ml of 2.5M (0.1mol) butyllithium (reactant) dropwise to 25g (0.1mol) of I-19-a (reactant) 11.2g (0.1mol) potassium tert-butoxide ( Reactant) in a solution of 250ml tetrahydrofuran (solvent), then dropwise add a solution of 13.5g (0.13mol) trimethyl borate (reactant) in 50ml tetrahydrofuran (solvent), stir the mixture at -60°C for 30 minutes, and heat up naturally At -20°C, add 50ml of water and 10ml of concentrated hydrochloric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com