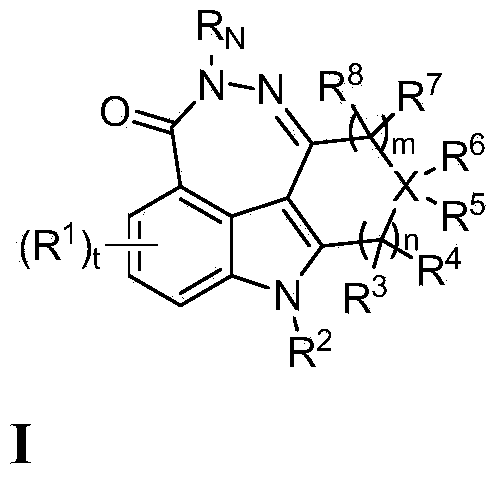
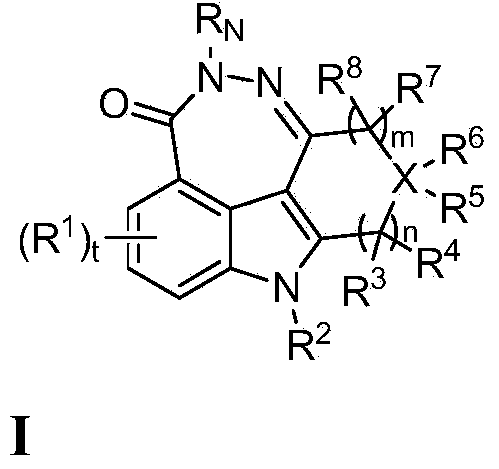
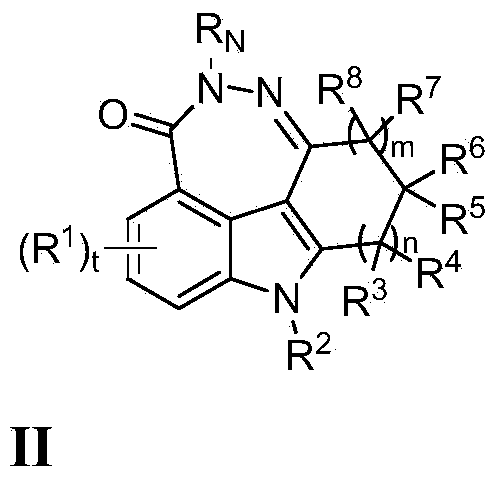
Fused tetra or penta-cyclic dihydrodiazepinocarbazolones as parp inhibitors
A technology of heterocyclyl and cycloalkyl, applied in the field of tetracyclic or pentacyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Example 1: Synthesis of Compounds 1-19.
[0204] Compound 1: 2,3,5,10-tetrahydro-[1,2]diazepine And[3,4,5,6-def]carbazol-6(1H)-one
[0205]
[0206] step 1: Methyl 2-bromo-3-((3-oxocyclohex-1-en-1-yl)amino)benzoate
[0207]
[0208] Methyl 3-amino-2-bromobenzoate (2.39 g, 10.0 mmol) and cyclohexane-1,3-dione (1.12 g, 10.0 mmol) were dissolved in acetic acid (10 mL) at 25 °C under nitrogen . The mixture was stirred at 80°C for 8 hours. The resulting solid was purified by column chromatography on silica gel (eluting with hexane and ethyl acetate) to give 2-bromo-3-((3-oxocyclohex-1-en-1-yl)amino)benzoic acid Methyl ester (2.46 g, 76%) as a tan foam. 1 H NMR (CDCl 3 -d 1 )δ7.53-7.55(m,2H),7.37(dd,1H,J=7.2,8.4Hz),6.34(broad singlet,1H),5.57(s,1H),3.95(s,3H),2.56 -2.59(m,2H),2.40-2.42(m,2H),2.08-2.11(m,2H). MS(ESI)m / e[M+1] + 324.0.
[0209] Step 2: 4-Oxo-2,3,4,9-tetrahydro-1H-carbazole-5-carboxylic acid methyl ester
[0210]
[0211] Methyl 2-bromo-3...
Embodiment 2
[0220] Embodiment 2: the synthesis of compound 20-21
[0221] Compound 20: 8-Oxo-3,4,8,9-tetrahydro-2,4,9,10-tetraazepine[def]fluorene -2(1H)-Benzyl carboxylate
[0222]
[0223] step 1: 3-((2-bromo-3-(methoxycarbonyl)phenyl)amino)-5-oxo-5,6-dihydropyridine-1(2H)- Benzyl carboxylate
[0224]
[0225] Methyl 3-amino-2-bromobenzoate (0.25 g, 1.1 mmol) and benzyl 3,5-dioxopiperidine-1-carboxylate (0.13 g, 0.55 mmol) were dissolved at 25 °C under nitrogen in acetic acid (10 mL). The mixture was stirred at 70°C for 8 hours. The resulting solid was purified by column chromatography on silica gel (eluting with hexane / ethyl acetate) to give 3-((2-bromo-3-(methoxycarbonyl)phenyl)amino)-5-oxo-5 , Benzyl 6-dihydropyridine-1(2H)-carboxylate (0.13 g, 51%) as a tan foam. 1 H NMR (CDCl 3 -d 1 )δ7.53-7.58(m,3H),7.42-7.48(m,5H),5.56(s,1H),5.16(s,2H),4.46(s,2H),4.13(s,2H),3.93 (s,3H). MS(ESI)m / e[M+1] + 459.0.
[0226] Step 2: 4-Oxo-3,4-dihydro-1H-pyrido[3,4-b]indole-2,5(...
Embodiment 3
[0236] Embodiment 3: the synthesis of compound 22-25
[0237] Compound 22: 10-Methyl-2,3,5,10-tetrahydro-[1,2]diazepine and [3,4,5,6-def]carbazole -6(1H)-one
[0238]
[0239] step 1: 9-Methyl-4-oxo-2,3,4,9-tetrahydro-1H-carbazole-5-carboxylic acid methyl ester
[0240]
[0241] To a solution of methyl 4-oxo-2,3,4,9-tetrahydro-1H-carbazole-5-carboxylate (0.27 g, 1 mmol) in THF (5 ml) was added at 0 °C under nitrogen Potassium tert-butoxide (0.12 g, 1.05 mmol). After the reaction mixture was stirred for 30 minutes, iodomethane (0.76 g, 5.0 mmol) was added. After 3 hours, the reaction mixture was concentrated to give a residue and partitioned between EtOAc (40ml) and 1N HCl (5ml). Shake and separate layers. The organic layer was washed with 1N HCl (2×80ml) and brine (2×10ml), washed with Na 2 SO 4 Drying, filtration and concentration gave a solid (0.46g). The solid was directly used in the next reaction without further purification. 1 H NMR (DMSO-d 6 )δ7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com