Porphyrin derivate with macrosubstituent, preparation process thereof and use as small molecule antioxidant
A technology of derivatives and substituents, which is applied in the fields of porphyrin derivatives with large substituents, their preparation and their application as small molecule antioxidants, which can solve the problems of limiting drug feasibility, inability to enter cells, short half-life, etc. problem, to achieve the effect of increasing practicability, treating cerebral thrombosis, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
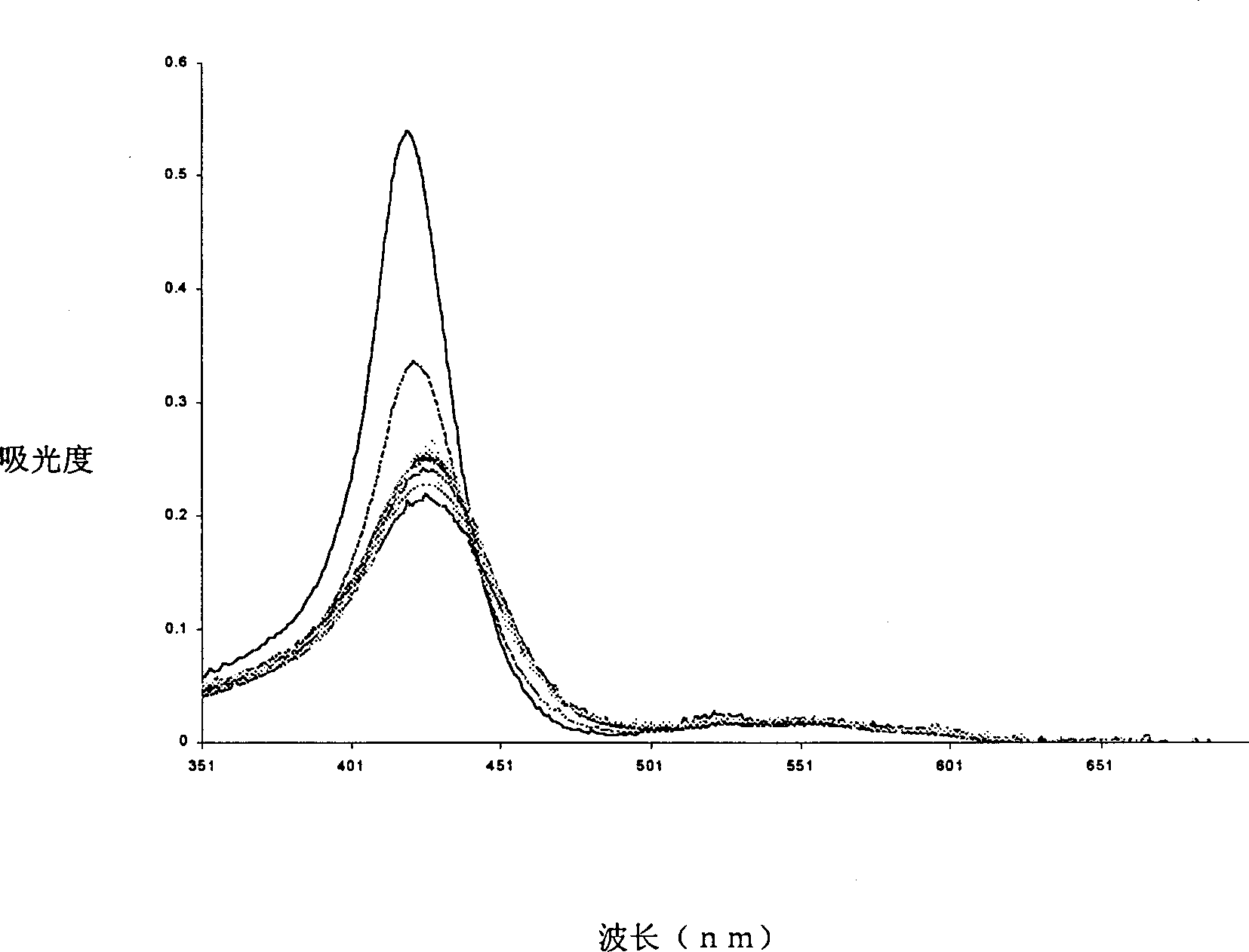
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of iron-5,10,15,20-tetra[(N-cyclohexyl)-2'-pyridyl]porphyrin pentachloride (structural formula 1)
[0045] 10.7 g of 2-pyridylcarbaldehyde was dissolved in 1 liter of propionic acid, and then 6.7 g of pyrrole was added. The resulting mixture was heated to reflux in air for 1 hour and then cooled to room temperature. Propionic acid was distilled off under vacuum. Silica gel column separation was carried out with chloroform to obtain 1.74 g of pure 5,10,15,20-tetrakis(2'-pyridyl)porphyrin. Yield 10%.
[0046] 1 gram of 5,10,15,20-tetrakis(2'-pyridyl)porphyrin was dissolved in 10 milliliters of chlorocyclohexane and refluxed for 4 hours at 60°C. After the reaction was complete, the solution was cooled to room temperature. Distill off excess chlorocyclohexane under vacuum to obtain 1.8 grams of 5,10,15,20-tetrakis[(N-cyclohexyl)-2'-pyridyl]porphyrin tetrachloride, yield 99% .
[0047] 1.8 grams of 5,10,15,20-tetrakis[(N-cyclohexyl)-2'-pyrid...
Embodiment 2
[0048] Embodiment 2: Copper-5,10,15,20-four [(N-cyclobutanyl)-3'-pyridyl] synthesis of porphyrin tetrachloride (structural formula 2)
[0049] 10.7 g of 3-pyridylcarbaldehyde was dissolved in 1 liter of propionic acid, and then 6.7 g of pyrrole was added. The resulting mixture was heated to reflux in air for 1 hour and then cooled to room temperature. Propionic acid was distilled off under vacuum. Silica gel column separation was carried out with chloroform to obtain 2.6 g of pure 5,10,15,20-tetrakis(3'-pyridyl)porphyrin. Yield 15%.
[0050] 2 grams of 5,10,15,20-tetrakis(3'-pyridyl)porphyrin was dissolved in 30 milliliters of chlorocyclobutane and refluxed for 4 hours at 60°C. After the reaction was complete, the solution was cooled to room temperature. Distill off excess chlorocyclobutane under vacuum to obtain 3.1 g of 5,10,15,20-tetrakis[(N-cyclobutanyl)-3'-pyridyl]porphyrin tetrachloride, yield 99% .
[0051] 3 grams of 5,10,15,20-tetrakis[(N-cyclobutyl)-3'-pyridyl]...
Embodiment 3
[0052] Embodiment 3: Cobalt-5,10,15,20-four [(N-cyclobutanyl)-4'-pyridyl] synthesis of porphyrin pentachloride (structural formula 3)
[0053] 10.7 g of 4-pyridylcarbaldehyde was dissolved in 1 liter of propionic acid, and then 6.7 g of pyrrole was added. The resulting mixture was heated to reflux in air for 1 hour and then cooled to room temperature. Propionic acid was distilled off under vacuum. Silica gel column separation was carried out with chloroform to obtain 4.3 g of pure 5,10,15,20-tetrakis(4'-pyridyl)porphyrin with a yield of 25%.
[0054] 4 grams of 5,10,15,20-tetrakis(4'-pyridyl)porphyrin was dissolved in 30 milliliters of chlorocyclobutane and refluxed at 60°C for 4 hours. After the reaction was complete, the solution was cooled to room temperature. Distill off excess chlorocyclobutane under vacuum to obtain 6.1 g of 5,10,15,20-tetrakis[(N-cyclobutanyl)-4'-pyridyl]porphyrin tetrachloride, yield 99% .
[0055] 6 grams of 5,10,15,20-tetrakis[(N-cyclobutyl)-4'-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com