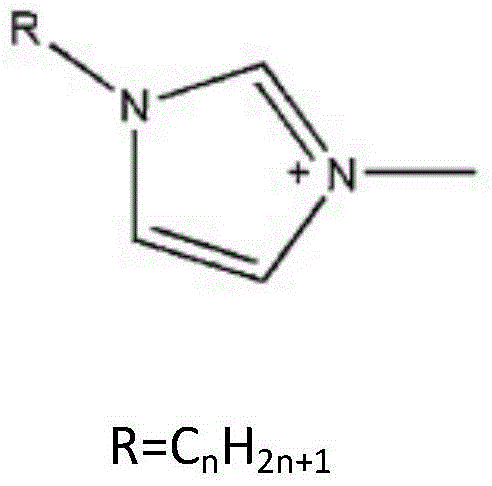
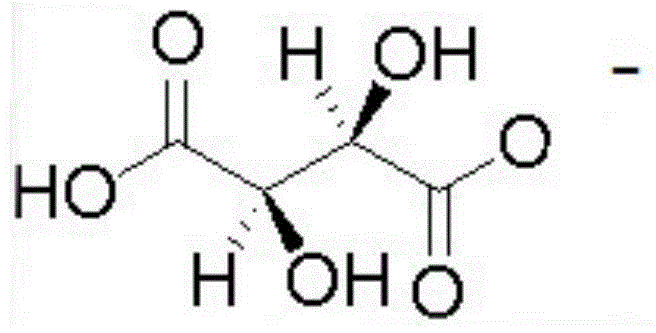
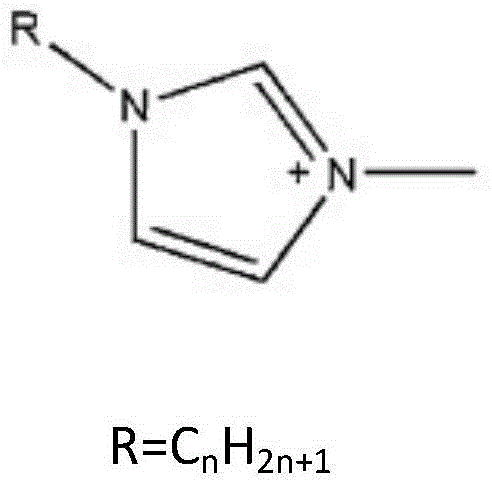
Reagent and method for splitting ofloxacin racemate by using ionic liquid and l-dibenzoyl tartaric acid
A ‐dibenzoyl tartaric acid, ionic liquid technology, applied in the field of chemical separation of chiral medicines, can solve the problems of difficulty in the separation of ofloxacin racemates and unfavorable industrial applications, and reduce the reagents required for separation , the effect of improving efficiency and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Ofloxacin racemate and 1-ethyl-3-methylimidazole L-tartrate were dissolved in deionized water to form 1-ethyl-3-methylimidazole L-tartrate with a concentration of 0.05mol / L , the concentration of ofloxacin racemate is an aqueous phase of 1000ppm, and simultaneously L-dibenzoyltartaric acid is dissolved in n-decyl alcohol to form an organic phase containing L-dibenzoyltartaric acid concentration of 0.15mol / L. Mix the organic phase and the aqueous phase with a volume ratio of 1:1 and put them in a shaker, shake for 2 hours, let stand, take the aqueous phase clear liquid to detect the concentration of L-ofloxacin and D-ofloxacin respectively, and the separation factor Reached 3.6.
Embodiment 2
[0021] Ofloxacin racemate and 1-ethyl-3-methylimidazole L-tartrate were dissolved in deionized water to form 1-ethyl-3-methylimidazole L-tartrate with a concentration of 0.05mol / L , the concentration of ofloxacin racemate is an aqueous phase of 1000ppm, and simultaneously L-dibenzoyltartaric acid is dissolved in n-decyl alcohol to form an organic phase containing L-dibenzoyltartaric acid concentration of 0.5mol / L. Mix the organic phase and the aqueous phase with a volume ratio of 1:1 and put them in a shaker, shake for 2 hours, let stand, take the aqueous phase clear liquid to detect the concentration of L-ofloxacin and D-ofloxacin respectively, and the separation factor reached 1.73.
Embodiment 3
[0023] Ofloxacin racemate and 1-ethyl-3-methylimidazole L-tartrate were dissolved in deionized water to form 1-ethyl-3-methylimidazole L-tartrate with a concentration of 0.05mol / L , the concentration of ofloxacin racemate is an aqueous phase of 1000ppm, and simultaneously L-dibenzoyltartaric acid is dissolved in n-decyl alcohol to form an organic phase containing L-dibenzoyltartaric acid concentration of 0.05mol / L. Mix the organic phase and the aqueous phase with a volume ratio of 1:1 and put them in a shaker, shake for 2 hours, let stand, take the aqueous phase clear liquid to detect the concentration of L-ofloxacin and D-ofloxacin respectively, and the separation factor reached 1.97.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com