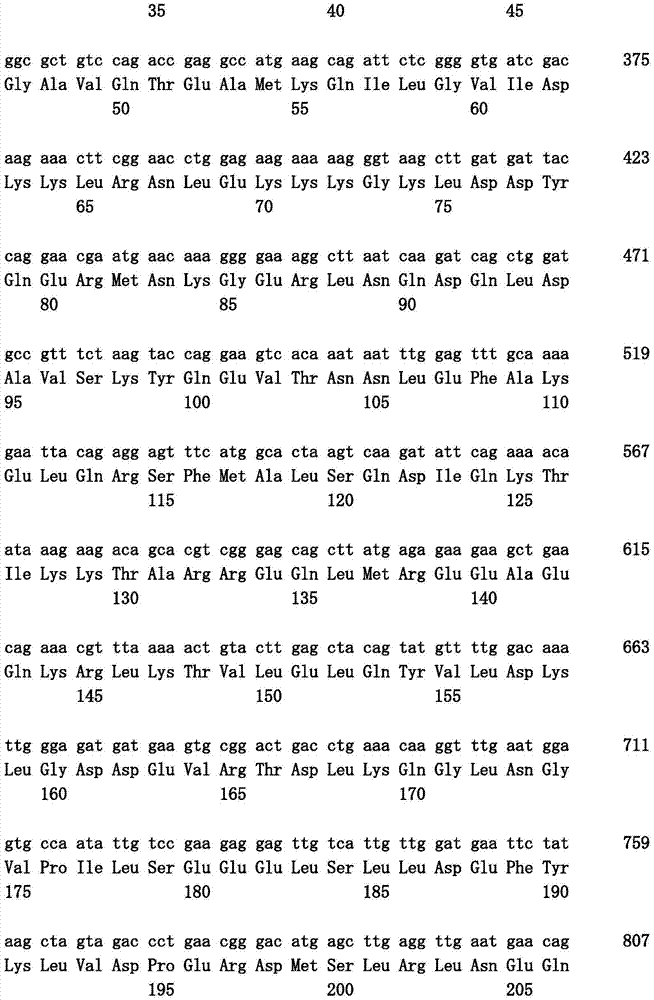
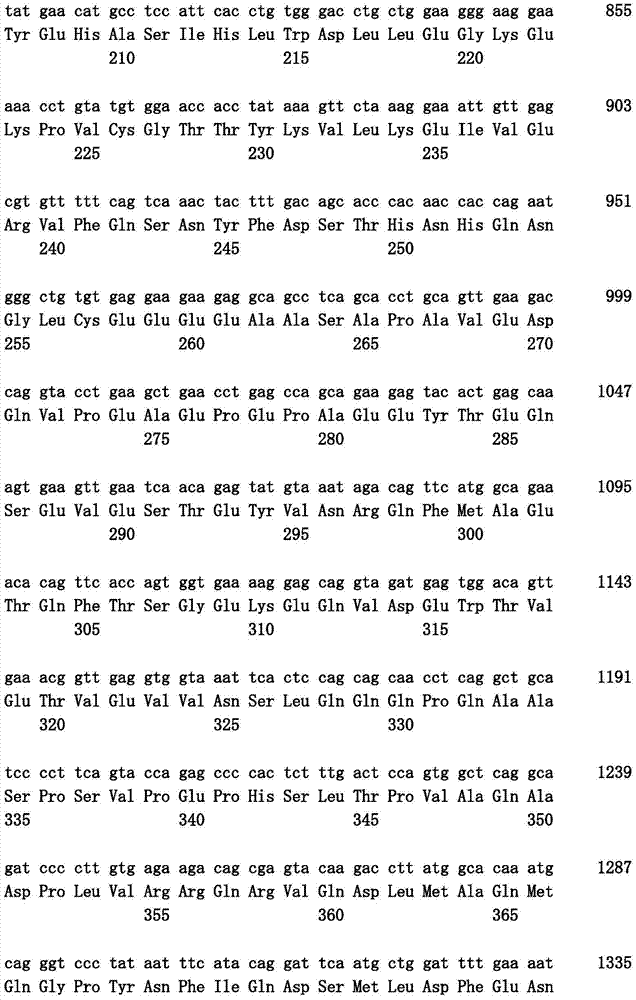
Pharmaceutical composition for treatment and/or prevention of pancreatic cancer
A composition and drug technology, applied in the directions of drug combinations, medical preparations containing active ingredients, anti-tumor drugs, etc., can solve the problems of not teaching CAPRIN-1 antigen protein, not recognizing CAPRIN-1 expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] [Example 1] Identification of pancreatic cancer antigen protein by SEREX method
[0199] (1) Preparation of cDNA library
[0200] Total RNA was extracted from the testis tissue of healthy dogs by the Acid guanidium-Phenol-Chloroform method (Acid guanidium-Phenol-Chloroform method), and using the Oligotex-dT30 mRNA purification kit (manufactured by Takara Shuzo Co., Ltd.), the polymer was purified according to the instructions attached to the kit. A RNA.
[0201] The thus obtained mRNA (5 µg) was used to synthesize a dog testis cDNA phage library. The cDNA phage library was prepared using cDNA Synthesis Kit, ZAP-cDNA Synthesis Kit, and ZAP-cDNA GigapackIII Gold Cloning Kit (manufactured by STRATAGENE), and the library was prepared according to the instructions attached to the kit. The size of the cDNA phage library produced was 7.73×10 5 pfu / ml.
[0202] (2) Screening of cDNA library using serum
[0203] The dog testis cDNA phage library prepared above was used for ...
Embodiment 2
[0211] [Example 2] Preparation of polyclonal antibody against human CAPRIN-1
[0212] Mix 1 mg of human CAPRIN-1 recombinant protein prepared according to Example 3 of WO2010 / 016526 with an equal volume of incomplete Freund's adjuvant (IFA) solution, administer it once every 2 weeks, and subcutaneously administer it to rabbits for 4 Second-rate. Blood is then collected to obtain antiserum containing polyclonal antibodies. This antiserum was further purified using a protein G carrier (manufactured by GE Healthcare Science) to obtain a polyclonal antibody against CAPRIN-1. In addition, an antibody was obtained by purifying the serum of a rabbit to which no antigen was administered using a protein G carrier in the same manner as above, and this obtained antibody was used as a control antibody.
Embodiment 3
[0213] [Example 3] Expression analysis of CAPRIN-1 protein in human pancreatic cancer
[0214] (1) Expression analysis of CAPRIN-1 protein on human pancreatic cancer cells
[0215] Whether or not CAPRIN-1 protein is expressed on the cell surface of four human pancreatic cancer cell lines (Capan-2, MIAPaCa-2, PANC-1, and BxPC-3) whose expression of the CAPRIN-1 gene was confirmed was examined. 10 cells of each human pancreatic cancer cell line whose gene expression was confirmed above were centrifuged in a 1.5 ml microcentrifuge tube. 6 cells. 2 μg (5 μl) of the CAPRIN-1-derived polyclonal antibody prepared in Example 2 was added, suspended in 95 μl of PBS containing 0.1% fetal bovine serum, and left to stand on ice for 1 hour. After washing with PBS, the suspension was suspended in 5 µl of FITC-labeled goat anti-rabbit IgG antibody (manufactured by Santa Cruz) and 95 µl of PBS containing 0.1% fetal bovine serum (FBS), and allowed to stand on ice for 1 hour. After washing wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com