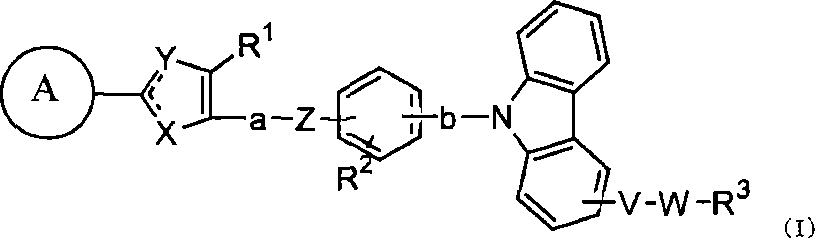
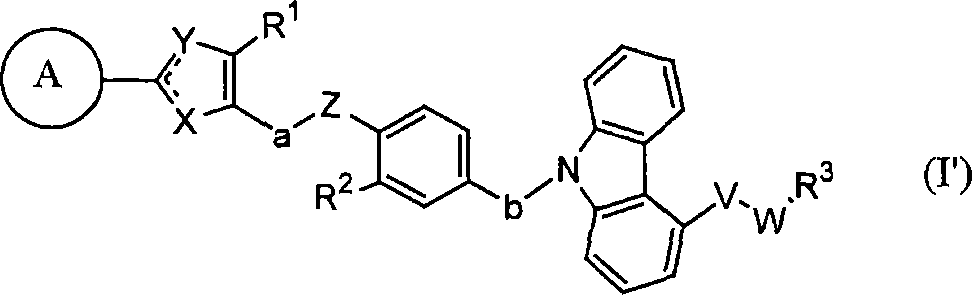
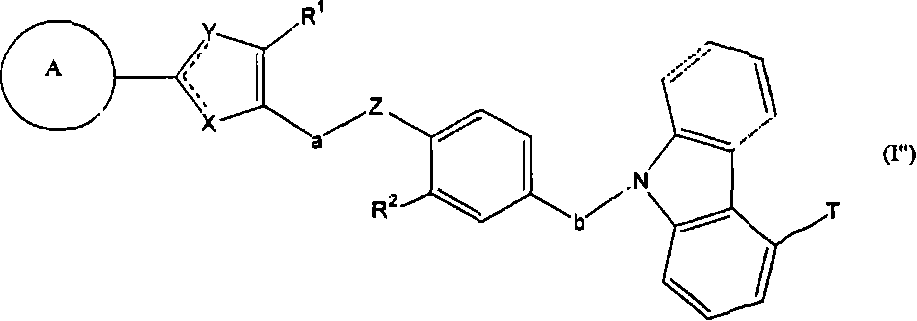
Carbazole derivative, solvate thereof, or pharmaceutically acceptable salt thereof
A technology of carbazole derivatives and solvates, which can be used in drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problems of reduced expression levels and promotion of obesity, and achieve excellent PPARγ inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0347] (2. The preparation method of the compound of the present invention)
[0348] The compound of the present invention represented by the general formula (I) can be produced, for example, according to the following A method and B method.
[0349] (2.1. The preparation method of the compound of the present invention-A method-)
[0350] An example of the method (Method A) for producing the compound of the present invention represented by the general formula (I) is described below. Method A is a method including the steps shown in the following process chart.
[0351]
[0352] In the above formula, A, V, W, X, Y, Z, a, b, R 1 , R 2 and R 3 means the same meaning as defined above. E represents a leaving group. Examples of E include hydroxyl, halogen, -OSO 2 R 7 (R 7 Methyl, trifluoromethyl, phenyl, tolyl or nitrophenyl may be mentioned. More specific examples of E include a chlorine atom or a bromine atom.
[0353] As described above, method A is a method for syn...
reference example 1
[0467] Synthesis of 4-((4-(chloromethyl)-2-methoxyphenoxy)methyl)-5-methyl-2-phenyloxazole
[0468]
[0469] Reference example 1(a)
[0470] Synthesis of 4,5-Dimethyl-2-phenyloxazole N-oxide
[0471]
[0472] 500 g of benzaldehyde and 476 g of diacetyl-methoxyme were suspended in 1 L of acetic acid, and ice-cooled. Hydrogen chloride gas was slowly bubbled in at an internal temperature of 7°C to make it saturated. Stir overnight at room temperature. The reaction solution was poured into 1.5 kg of ice, and neutralized with a 25% aqueous sodium hydroxide solution. The precipitated crystals were filtered off, and washed successively with 1 L of water and 1 L of diisopropyl ether. The obtained substance was dissolved in 3 L of chloroform, and the insoluble matter was filtered off. The filtrate was dried over 200 g of anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. 3 L of IPE was added to the residue to crystallize it, and th...
reference example 2
[0490] Synthesis of 4-((4-(chloromethyl)-2-methoxyphenoxy)methyl)-2-(furan-2-yl)-5-methyloxazole
[0491]
[0492] Using furfural instead of benzaldehyde used in Reference Example 1(a), the same operations as in Reference Examples 1(a) to 1(d) were carried out to obtain the title compound.
[0493] 1 H-NMR (400MHz, DMSO-d 6 )δppm: 2.41 (3H, s) 3.76 (3H, s) 4.72 (2H, d) 4.97 (2H, s) 6.71 (1H, dd) 6.98 (1H, dd) 6.71 (1H, dd) 6.98 (1H, dd )7.06(1H,d)7.08(1H,d)7.11(1H,dd)7.91(1H,dd)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com