Human monoclonal antibody specific for lipopolysaccarides (LPS) of serotype IATS 01 of pseudomonas aeruginosa
A human monoclonal antibody, Pseudomonas aeruginosa technology, applied in the direction of antibodies, DNA/RNA fragments, specific peptides, etc., can solve the problems of lack of protection, lack of effector function, etc., and achieve excellent results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
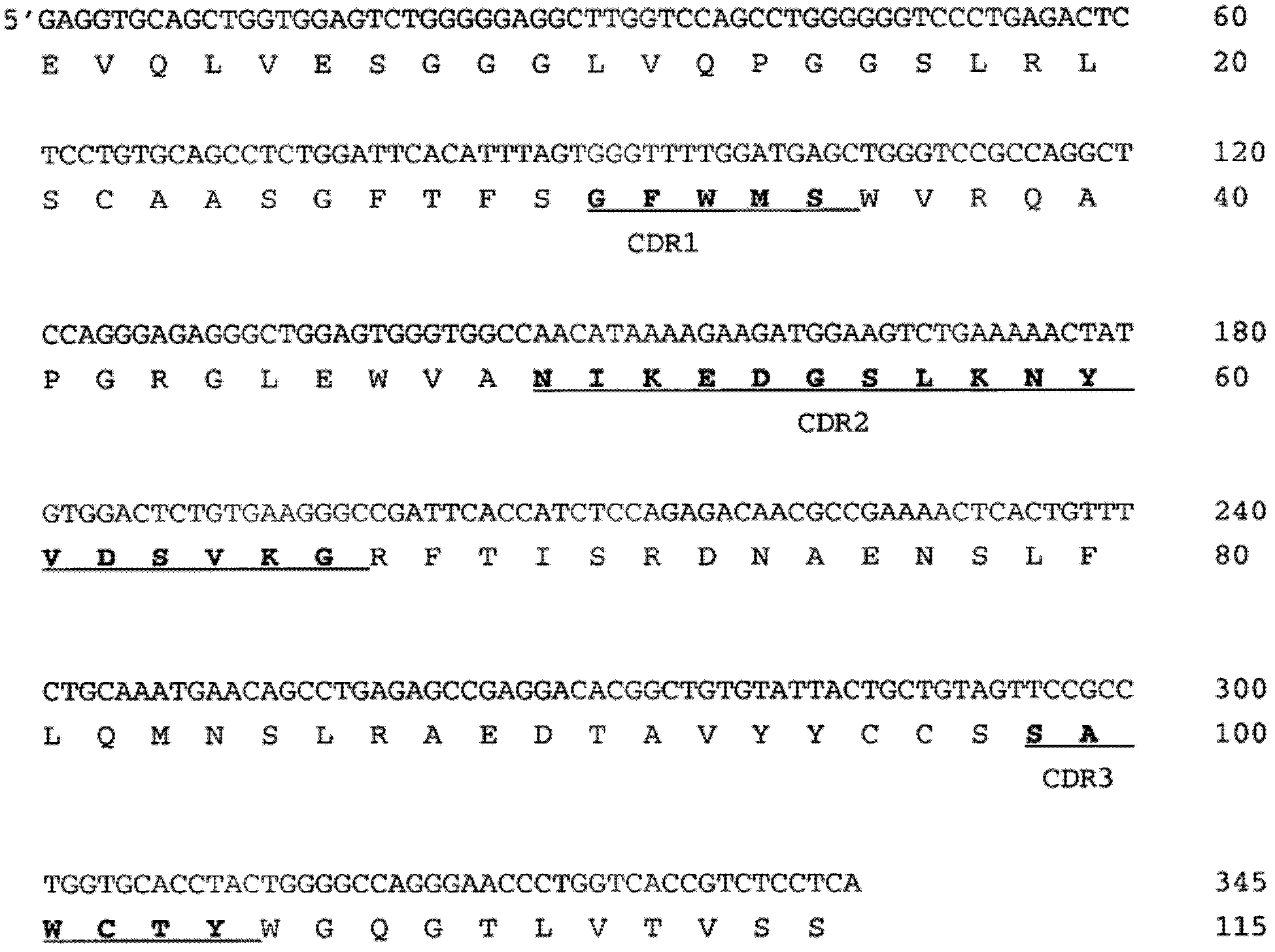
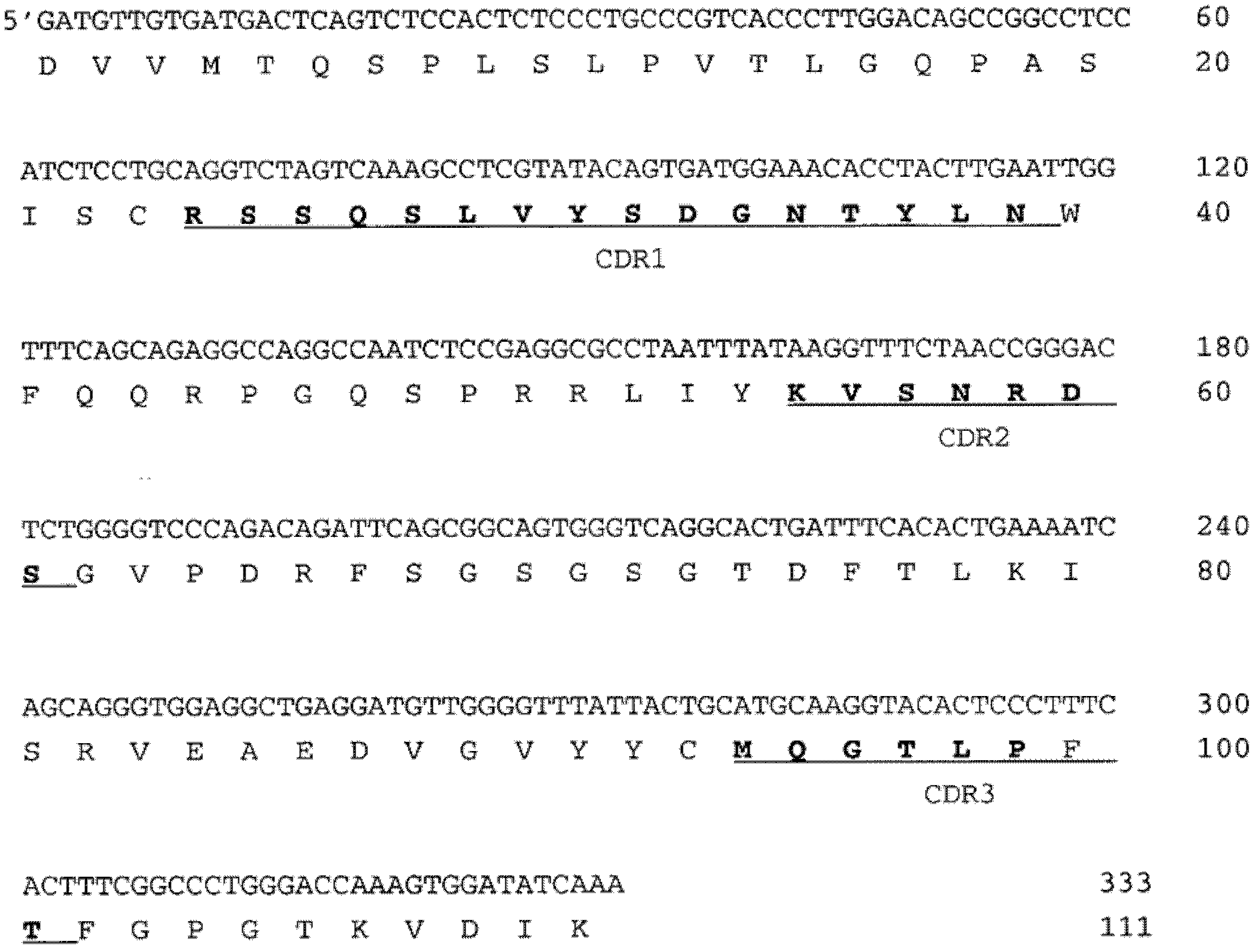
[0119] Example 1: DNA and amino acid sequence of 216-O1
[0120] The antibody specificity was determined by DNA sequence and amino acid sequence respectively. Determine the DNA sequence of the variable fragments of the heavy and light chains. In short, all RNA of hybridoma cells is isolated and reverse transcribed into complete cDNA. Using Cκ and Cμ-specific primers combined with the forward primer in the leader sequence, the IgM and κ variable regions and part of the constant regions were amplified by PCR. The PCR fragment was then removed by cutting out from the agarose gel and used as a template for sequencing with the primers described in Table III.
[0121] Table III
[0122] For 216-O1 IgM heavy chain and kappa light chain
[0123] Variable region PCR-primers for amplification and sequencing
[0124] Primer
[0125] Then make the sequence of the variable region and Vbase Index ( http: / / vbase.mrc-cpe.cam.ac.uk / )Compare. The comparison with the germline sequence shows that...
Embodiment 2
[0126] Example 2: Recognition of LPS isolated from Pseudomonas aeruginosa and clinical isolates of Pseudomonas aeruginosa serotype IATS O1 by monoclonal antibody 216-O1
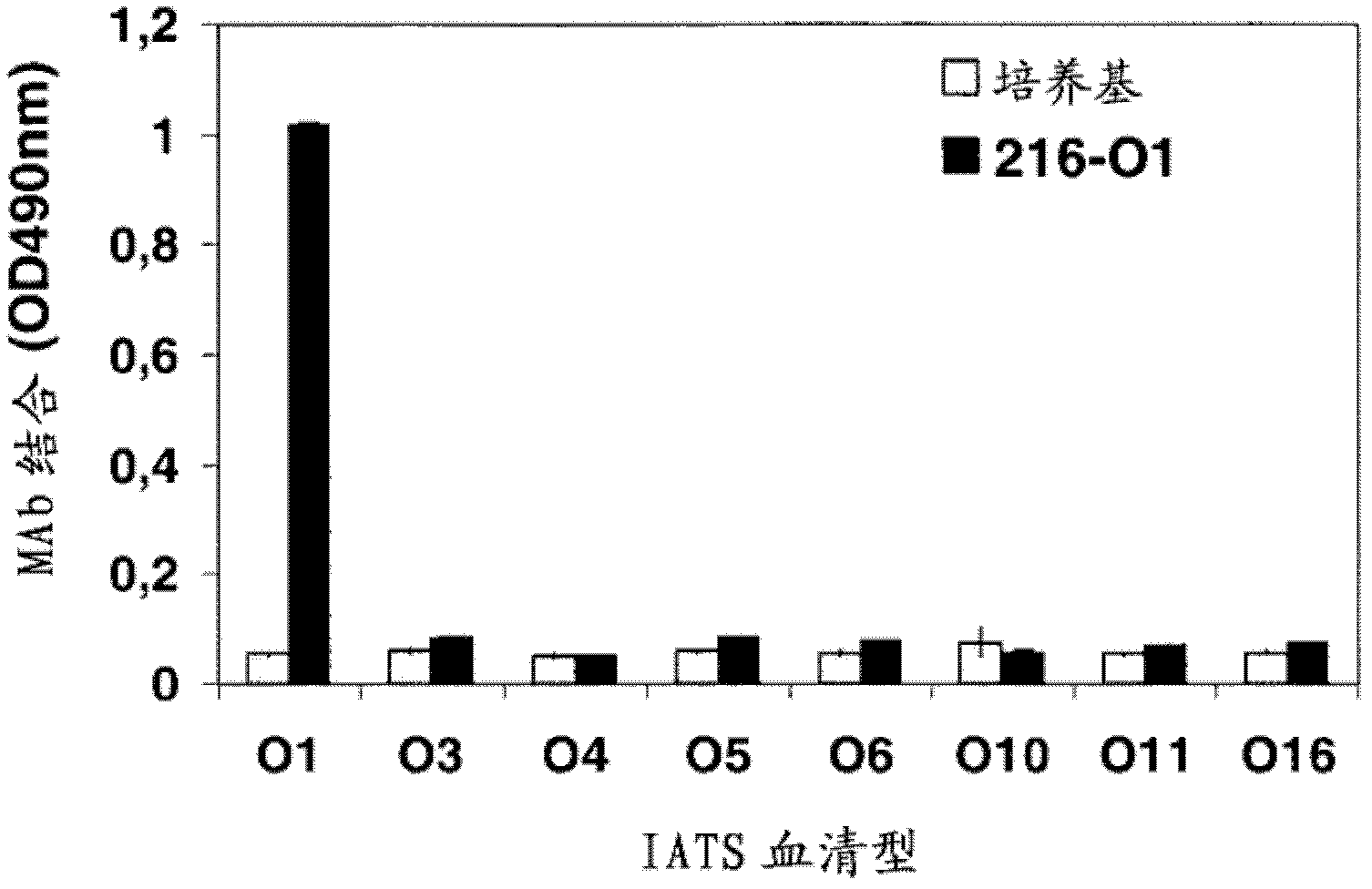
[0127] By immunizing healthy volunteers with the octavalent O-PS-toxin A vaccine, 216-O1 was produced. The vaccine contains LPS of IATS O1 strain PA53. To determine the specificity of LPS, 216-O1 was tested on a series of LPS isolated from Pseudomonas aeruginosa (Table 1) ( image 3 ). In order to study whether 216-O1 specifically recognizes IATS O1 Pseudomonas aeruginosa, it was tested on 17 reference strains ( Figure 4a ).
[0128] In addition, different clinical isolates of serotype IATS O1 were tested by whole-cell ELISA ( Figure 4b ) Binding to 216-O1 and other anti-Pseudomonas aeruginosa LPS IATS O1 antibodies (MAb C1 and MAb C2). The serotypes of all isolates were determined using a commercially available serotype agglutination kit and confirmed by PCR. 216-O1 reacted specifically with the isolated LPS...
Embodiment 3
[0129] Example 3: In vitro activity of 216-O1: opsonizing phagocytic activity
[0130] The in vitro biological activity of 216-O1 was evaluated by the opsonized phagocytosis experiment based on flow cytometry. In the presence of normal rabbit serum as a source of complement, the fluorescent-labeled ((5(6)-FAM SE)-conjugated Pseudomonas aeruginosa of serotype IATS O1 was incubated with serially diluted 216-O1. Conditioned Bacteria were incubated with differentiated HL-60 cells (promyelocytic cell line, ATCC: CCL-240; differentiation into phagocytes was achieved by adding 0.1M dimethylformamide for 4 days). Phagocytosis was opsonized by FACS analysis. In the presence of active complement (heat-inactivated serum), the green fluorescence of HL-60 cells is analyzed and compared with the background staining of (5(6)-FAM SE)-binding bacteria containing HL-60 cells to determine Positive opsonizing phagocytic activity. The average results of 2 independent experiments are shown in Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com