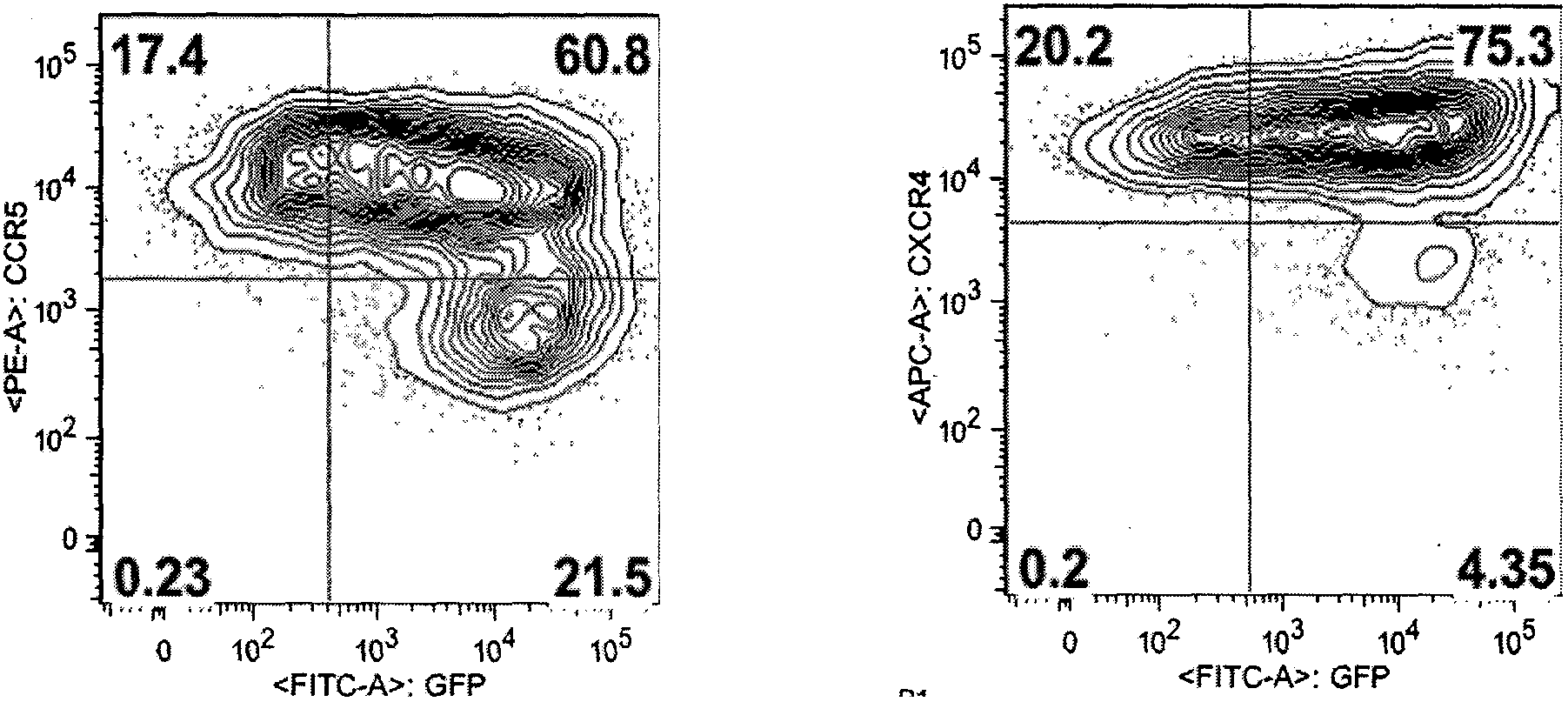
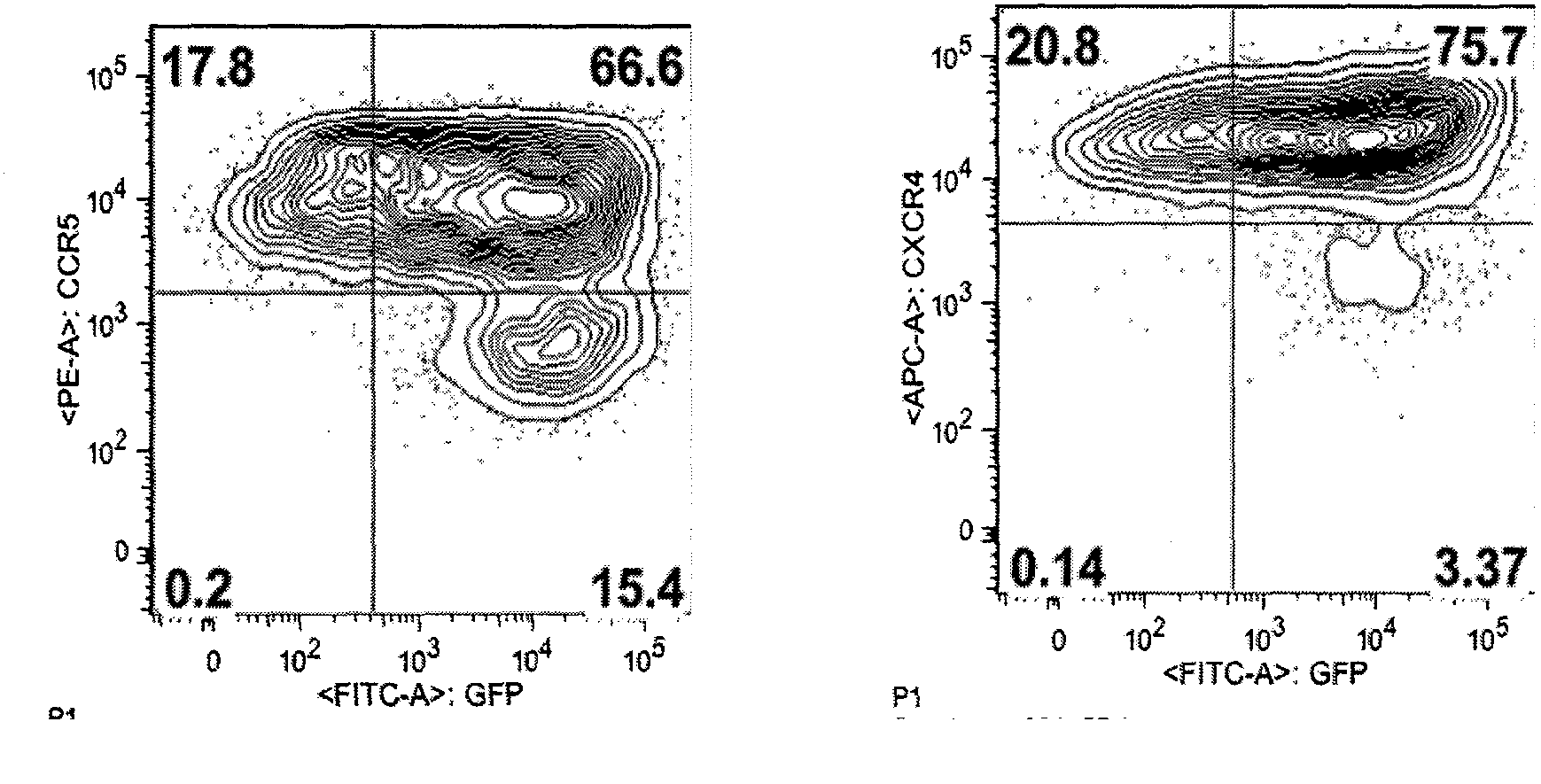
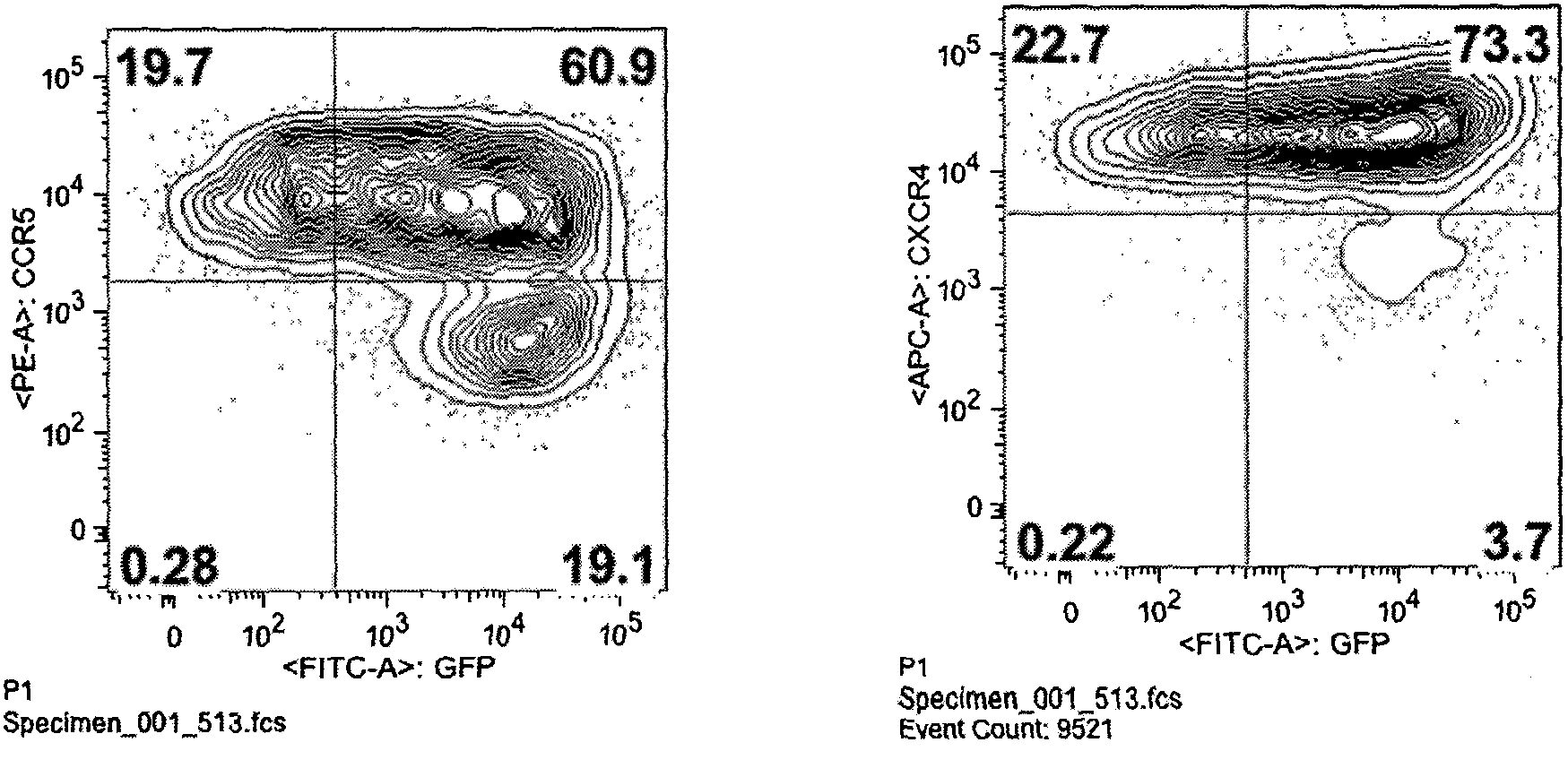
Fusion proteins for regulating and controlling CCR5 and CXCR4 genes and regulation and control method
A technology of gene activity and species, applied in the field of fusion proteins, can solve problems such as hindering application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0071] Example 1 TALEN plasmid amplification;
[0072] First, take 50 μl of competent cells DH5α (TIANGEN) from the -80°C refrigerator, and thaw on ice for about 10 minutes. Then add TALEN plasmid DNA to the competent cell suspension (50ul competent cells can be saturated by 500nG supercoiled DNA), gently rotate the centrifuge tube to mix the contents, and let it stand in an ice bath for 30min. Immediately after the heat shock in a water bath at 42°C for 90 seconds, place it on ice to cool for 3-5 minutes quickly after the heat shock, then add 500ul LB liquid medium (without antibiotics) to the tube, shake well and take 100μl to spread on On the screening plate containing Amp, place it face up for half an hour. After the bacterial solution is completely absorbed by the medium, invert the culture dish and incubate at 37°C for 16 hours.
[0073] Pick a single clone on the culture plate and put it in 3ml Amp LB medium, and culture it in a shaker at 37°C; after 16 hours, inoculat...
example 2
[0074] Example 2TALEN plasmid extraction (using Plasmid Plus Maxi)
[0075] Do the following preparations first. Add RNase A enzyme to P1 buffer, mix, and store at 2-8°C; then add RNase A in a ratio of 1:1000 Check whether solution P2 contains SDS precipitate. If so, place it at 37°C for a short time until it is completely dissolved; before use, add ethanol (96-100%) to solution PE (see bottle label).
[0076] Next, a large amount of plasmid extraction is performed. Collect 100 mL of Escherichia coli liquid obtained by overnight shaking with a centrifuge at 4°C and 6000×G for 15 minutes. Then resuspend the bacteria with 5ml of solution P1 and mix them repeatedly with a shaker. Then add 5ml of solution P2, and gently invert the bottle up and down 4-6 times until the bacteria are completely lysed and appear sticky. The lysed solution was then placed at room temperature for 3 min. If LyseBlue reagent has been added, the cell suspension will turn blue. Put the new QIAfilt...
example 3
[0077] Example 3Ghost cell cell culture
[0078] Before the cells were passaged, the cells were observed under the microscope to cover the bottom of the culture dish. Then put the cell culture medium (DMEM), PBS, and trypsin at room temperature for half an hour, and at the same time irradiate the cells with ultraviolet rays for 30 minutes before experimenting on the ultra-clean bench.
[0079] First use a pipette to suck away the original culture solution in the overgrown single-layer Ghost cell culture bottle, add 1ml of 0.25% trypsin solution to digest the cells (the volume added depends on the size of the specific culture dish. For example, a 10cm culture dish The cultured cells can be digested with 5ml 0.25% trypsin solution), and placed in a 37°C incubator to digest the cells for about 5 minutes; observe the changes of cell adhesion and shape under an inverted microscope, when the original adherent cells gradually tend to be round , floated up and began to disperse into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com