Method for recycling fluorides in preparation process of silicon-containing compound
A technology of silicon compounds and fluorides, applied in the direction of silicon halide compounds, silicon oxide, silicon dioxide, etc., can solve problems such as the inability to achieve the recycling of fluorides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
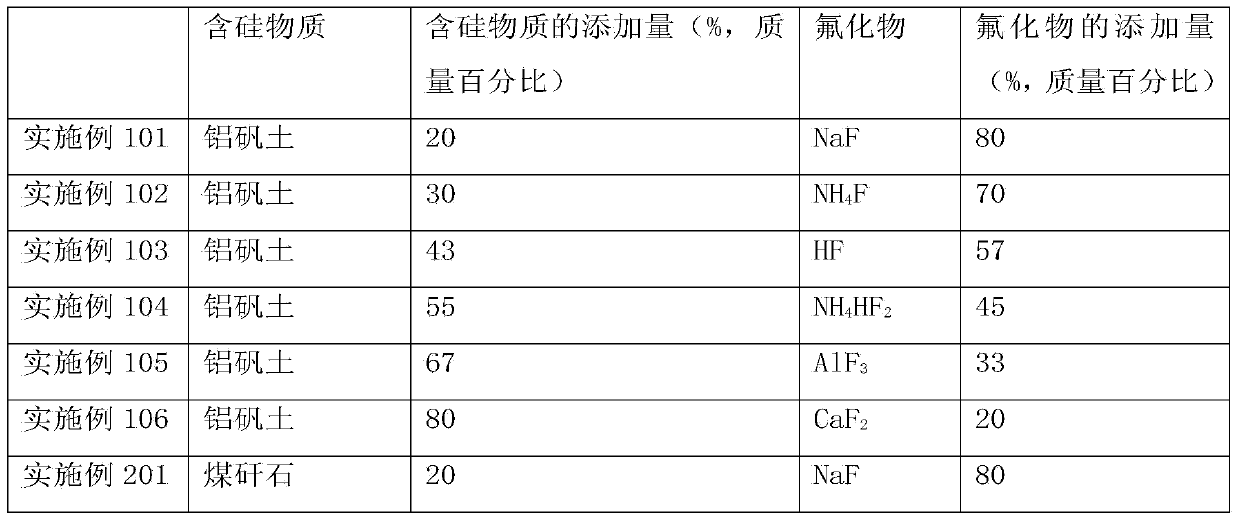
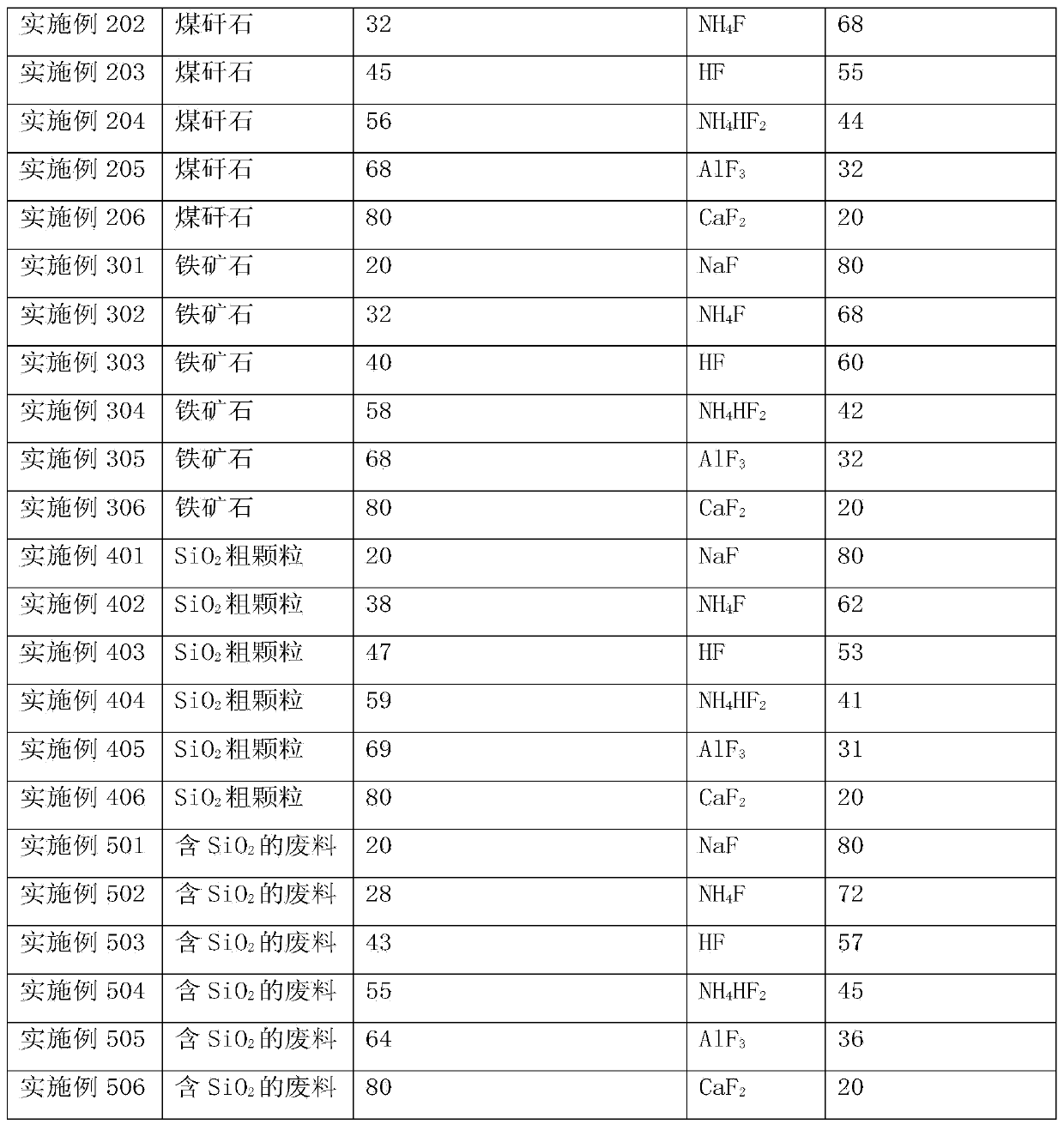
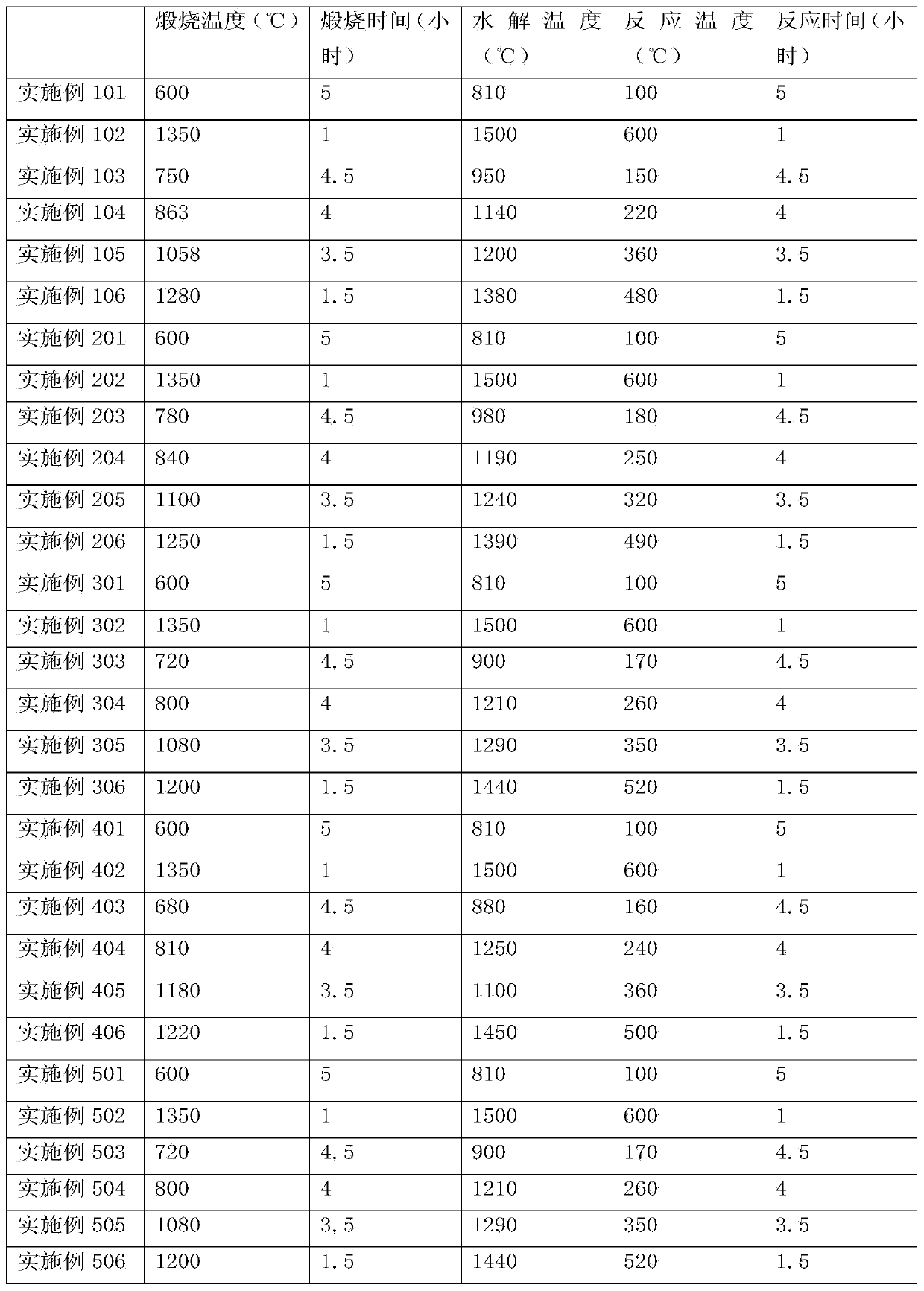
[0057] Embodiment 1: Preparation of high-purity SiF 4 CaF 2 recycling
[0058] Will contain SiO 2 waste with CaF 2 Mix well to get a mixture, SiO 2 with CaF 2 The particle size is below 3mm, the CaF in the resulting mixture 2 The mass percentage is 80%. Calcinate the mixture at a temperature of 900-1100°C for 1 hour to generate CaO and SiF 4 , SiF 4 It is gaseous at high temperature and escapes from solid reactants. SiF that will escape 4 Introduce into another reactor and hydrolyze at a temperature of 810-1000°C to form SiO 2 and HF gas. Introduce the HF gas produced by hydrolysis into the reactor, and react with the reaction product CaO at 300-600°C for 2.5 hours to obtain CaF again 2 , thus realizing CaF 2 of reuse. The reaction equation is as follows:
[0059] SiO 2 +2CaF 2 →2CaO+SiF 4 (4)
[0060] SiF 4 +2H 2 O→SiO 2 +4HF (5)
[0061] CaO+2HF→CaF 2 +H 2 O (6)
Embodiment 2
[0062] Embodiment 2: Preparation of high-purity SiF 4 Process AlF 3 recycling
[0063] Bauxite ((Al 2 o 3 ) m (TiO 2 ) nSiO 2 ) with AlF 3 Mix well to get a mixture, bauxite and AlF 3 The particle size is below 5mm, and the AlF in the resulting mixture 3 The mass percentage is 60%. Calcinate the mixture at a temperature of 1100-1350°C for 3 hours to generate Al 2 o 3 、H 2 O and SiF 4 . SiF 4 Escape from solid reactant. SiF that will escape 4 Introduce into another reactor and hydrolyze at a temperature above 1000-1200°C to form SiO 2 and HF gas. The HF gas produced by hydrolysis is introduced into the reactor, and the reaction product Al 2 o 3 After reacting at 100°C for 5 hours, AlF was recovered 3 , thus achieving AlF 3 of reuse. The reaction equation is as follows:
[0064] 3SiO 2 +4AlF 3 ·3H 2 O→2Al 2 o 3 +3SiF 4 +12H 2 O (7)
[0065] SiF 4 +2H 2 O→SiO 2 +4HF (8)
[0066] al 2 o 3 +6HF→2AlF 3 +3H 2 O (9)
Embodiment 3
[0067] Embodiment 3: Preparation of nano-SiO 2 NH in process 4 HF 2 efficient use of
[0068] SiO 2 Coarse particles and NH 4 HF 2 Mix well, SiO 2 with NH 4 HF 2 The particle size is below 7mm, and the NH in the resulting mixture 4 HF 2 The mass percentage is 40%. Calcinate the mixture at a temperature of 600-700°C for 5 hours to generate NH 3 、H 2 O and SiF 4 , SiF 4 escaped from the solid. SiF that will escape 4 Introduce into another reactor and hydrolyze at a temperature of 1200-1300°C to form SiO 2 and HF gas. The HF gas produced by hydrolysis is introduced into the reactor, and the reaction product NH 3 and H 2 O was reacted at 150°C for 4.5 hours to regain NH 4 HF 2 , thus achieving NH 4 HF 2 of reuse. The reaction equation is as follows:
[0069] SiO 2 +2NH 4 HF 2 →SiF 4 +2NH 3 +2H 2 O (10)
[0070] SiF 4 +2H 2 O→SiO 2 +4HF (11)
[0071] NH 3 ·H 2 O+2HF→NH 4 HF 2 +H 2 O (12)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com