Leukemia fusion gene fluorescence PCR (Polymerase Chain Reaction) detection kit
A technology that integrates genes and kits, and is applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., and can solve the problems of not setting positive internal controls, not preventing PCR product contamination, and being unable to monitor false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment provides a PCR detection kit for leukemia fusion genes BCR / ABL, TEL / AML1, and AML1 / ETO.
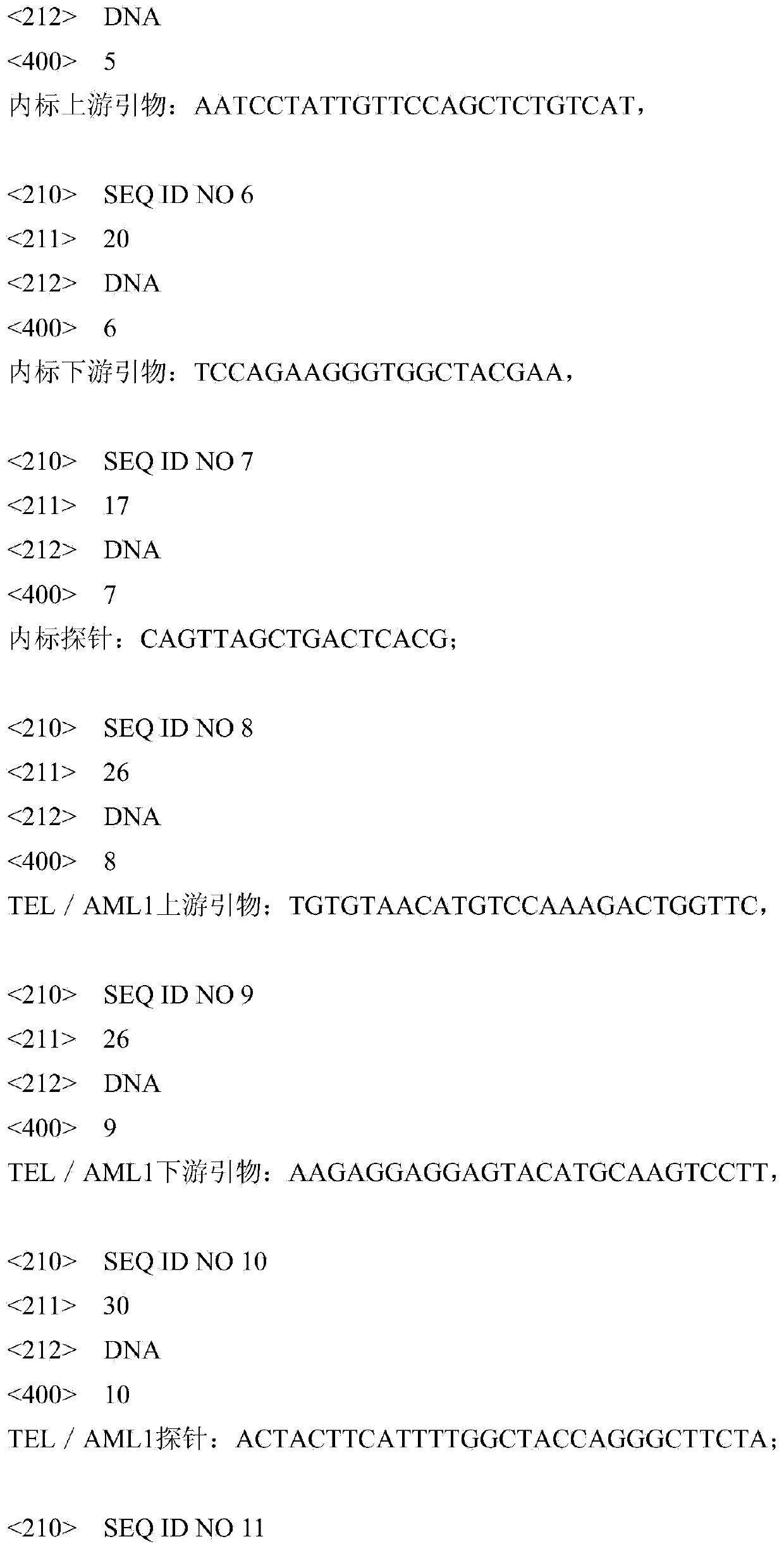
[0035] ①Internal standard (positive internal control): It is a recombinant of a 100-base-pair artificially synthesized DNA sequence inserted into the pUC18T vector, that is, a plasmid, with a concentration of 1.00E+04copies / ml~5.00E+04copies / ml, used as Positive internal control in the PCR amplification system to prevent false negatives caused by possible PCR interference substances in the sample; the 100 base pair sequence is as follows: 5'-TAGGACTTAAATCCTATTGTTCCAGCTCTGTCATCCAGTTAGCTGAC TCACGTATTCGTAGCCACCCTTCTGGAGGTGCAATCTCAATTATGTCATCAGG-3';
[0036] ②PCR reaction solution: 5 μl of 10×PCR reaction buffer, 0.2 mmol / L deoxyribonucleoside triphosphate, 0.2 μmol / L~0.4 μmol / L upstream and downstream primers for target polynucleotide amplification, 0.2 μmol / L ~0.4μmol / L probe for target polynucleotide detection, 0.2μmol / L~0.4μmol / L upstream and downstream primers for ...
Embodiment 2
[0054] This example provides the operation steps of the leukemia fusion gene BCR / ABL, TEL / AML1, and AML1 / ETO detection kits in Example 1 for detecting unknown samples such as patient bone marrow tissue.
[0055] 1. Reagent preparation:
[0056] According to the number of samples to be tested, negative control and positive control, take the corresponding amount of PCR reaction solution (38μl / person), enzyme mixture (2μl / person) and internal standard (0.5μl / person) in proportion, fully Mix well to form a PCR-mix, centrifuge briefly and set aside.
[0057] 2. Sample processing and sample addition
[0058] 1) Add 5 μl each of the same sample to be tested, negative control, and positive control to 5 different PCR reaction tubes;
[0059] 2) After standing for 10 minutes, add 45 μl of PCR-mix to each tube, pipette and mix 2-3 times, cover the tube cap (after removing air bubbles), and centrifuge at 2000 rpm for 30 seconds.
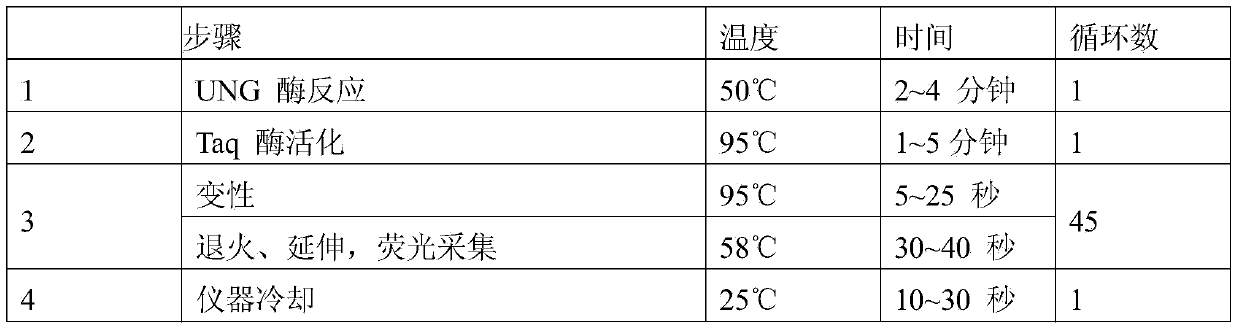
[0060] 3. Perform PCR amplification on the fluorescent ...
Embodiment 3
[0069] This embodiment provides another PCR detection kit for leukemia fusion genes BCR / ABL, TEL / AML1, and AML1 / ETO, which can be used to specifically distinguish whether each of the above three fusion genes is negative or positive.
[0070] Among components ①~⑤ of the kit, except for component ②, the other components are the same as those in Example 1.
[0071]Component 2. PCR reaction solutions in this embodiment are three kinds of PCR reaction solutions. Specifically, the first PCR reaction solution contains the primer and probe sequences of the leukemia fusion gene BCR / ABL and the primer-probe sequence of the internal standard. The second PCR reaction solution contains the primer and probe sequences of the leukemia fusion gene TEL / AML1 and the primer probe sequence of the internal standard, while the third PCR reaction solution contains the primer and probe sequences of the leukemia fusion gene AML1 / ETO and internal standard primer probe sequences.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com