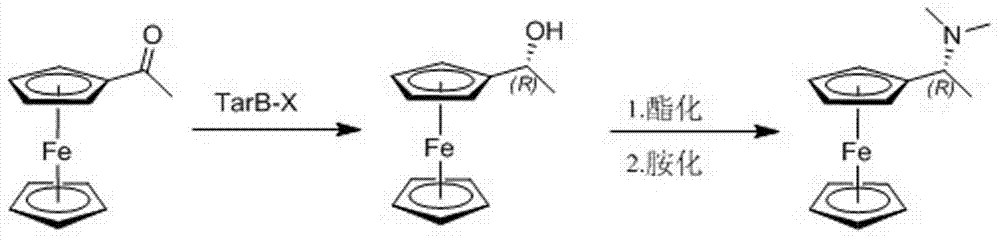
Preparation process for chiral (R)-1-ferrocenyl ethyl dimethylamine
A ferrocenyl ethyl dimethylamine and preparation technology technology, applied in the field of preparation technology of 1-ferrocenyl ethyl dimethylamine, can solve problems such as low yield and complicated operation process, and achieve yield High efficiency, simple operation process, and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation process embodiment 1 of a kind of chiral (R)-1-ferrocenyl ethyl dimethylamine of the present invention, its preparation step comprises successively:
[0022] Step 1, 11.4g (0.05mol) acetylferrocene and 250ml concentration are the tetrahydrofuran (THF) solution of the TarB-H of 0.4mol / L (0.1mol) adding the volume with magnetic stirring, low temperature thermometer and drying tube is In a 500ml three-neck flask, stir in ice bath to 0°C for 15min, add 3.8g (0.1mol) sodium borohydride (NaBH 4 ), stirred at room temperature for 2h, then transferred the reaction solution into a beaker with a volume of 1L, slowly added 300ml of hydrochloric acid (HCl) with a concentration of 1mol / L to quench the reaction, added 28g of sodium hydroxide (NaOH) solid to adjust the pH value to 12, and continue to stir for 30min, stand still for liquid separation, extract the aqueous phase with n-hexane (200ml×3), combine the organic phases, wash with saturated aqueous sodium chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com