A serum microRNA marker related to the occurrence of human fetal growth restriction and its application
A technology of growth restriction and markers, applied in the fields of genetic engineering and clinical medicine, can solve problems that have not received corresponding attention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 Research Object Selection and Grouping Basis
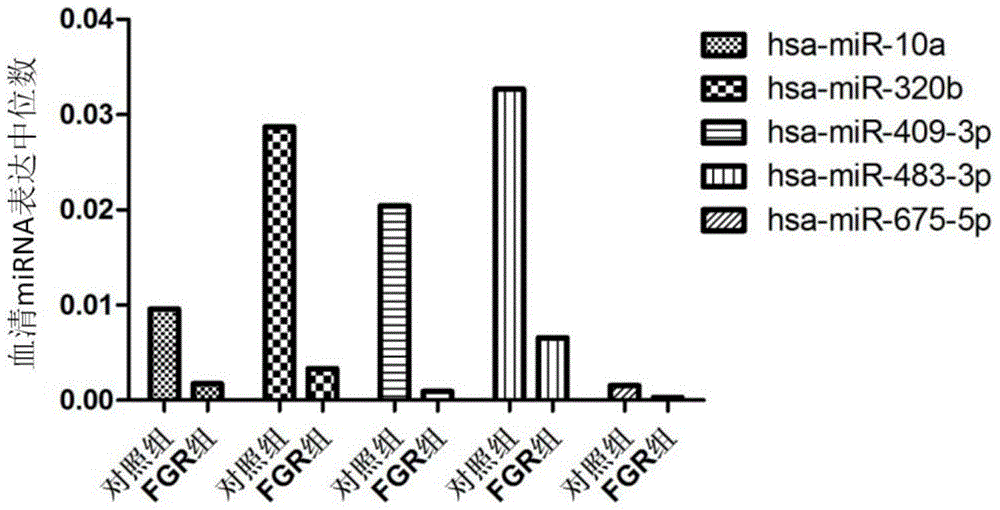
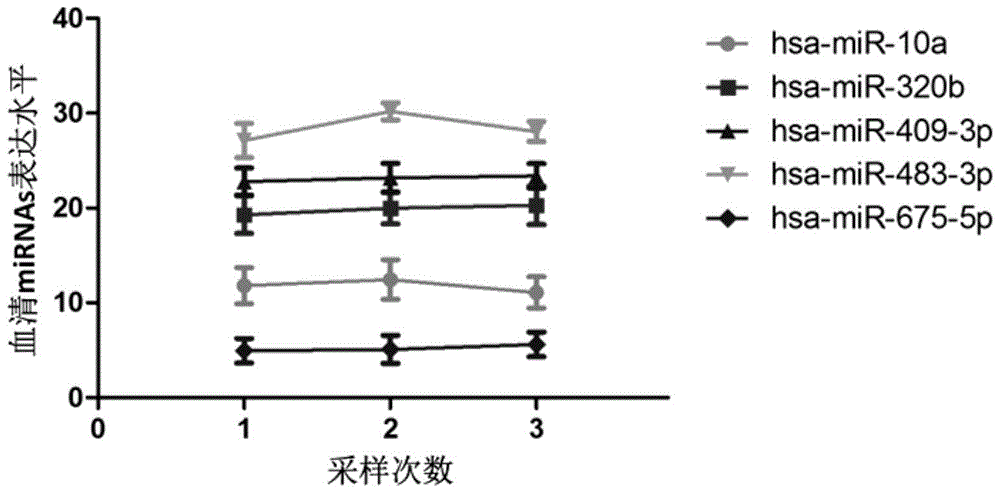
[0077] From July 2010 to January 2013, the inventor collected blood samples from fetal growth restriction cases and healthy control pregnant women who met the requirements from Huaian First People's Hospital Affiliated to Nanjing Medical University. A total of 80 healthy controls (average age of pregnant women: 26.39±3.42 days) and 80 cases of fetal growth restriction (average age of pregnant women: 27.47±4.15 days) were selected as subjects for Real-timePCR detection of miRNA expression. Specific sample classification criteria are as follows:
[0078] Group A: healthy control group (n=80, 10 people for microarray screening, 30 people for first-stage verification, 40 people for independent population verification):
[0079] 1. No other major systemic diseases.
[0080] Group B: fetal growth restriction group (n=80, 10 people for microarray screening, 30 people for phase one verification, 40 people for indepen...
Embodiment 2
[0083] Embodiment 2TaqManmiRNAarray screening
[0084] Preparation of cDNA samples: a) Take 100 μl of serum; b) Add 60 μl of lysate, vortex for 5 seconds, and let stand at room temperature for 3 minutes; c) Add 20 μl of protein solution, vortex for 5 seconds, stand at room temperature for 1 minute, and centrifuge at 11,000 g for 3 minutes; d) Take the above Clear to a new 2ml collection tube, add 270μl isopropanol, and vortex for 5s; e) Put the microRNA spin column in the collection tube, add the liquid in step d to the spin column, let stand at room temperature for 2min, centrifuge at 11000g for 30s, pour Discard the liquid in the collection tube and put the spin column back into the collection tube; f) Add 100 μl of eluent 1 to the microRNA spin column, centrifuge at 11,000 g for 30 seconds, discard the liquid in the collection tube, and put the spin column back into the collection tube; g) Add 700 μl Put the eluent 2 into the microRNA spin column, centrifuge at 11000g for 3...
Embodiment 3
[0098] Embodiment 3Real-timePCR method measures serum miRNA expression
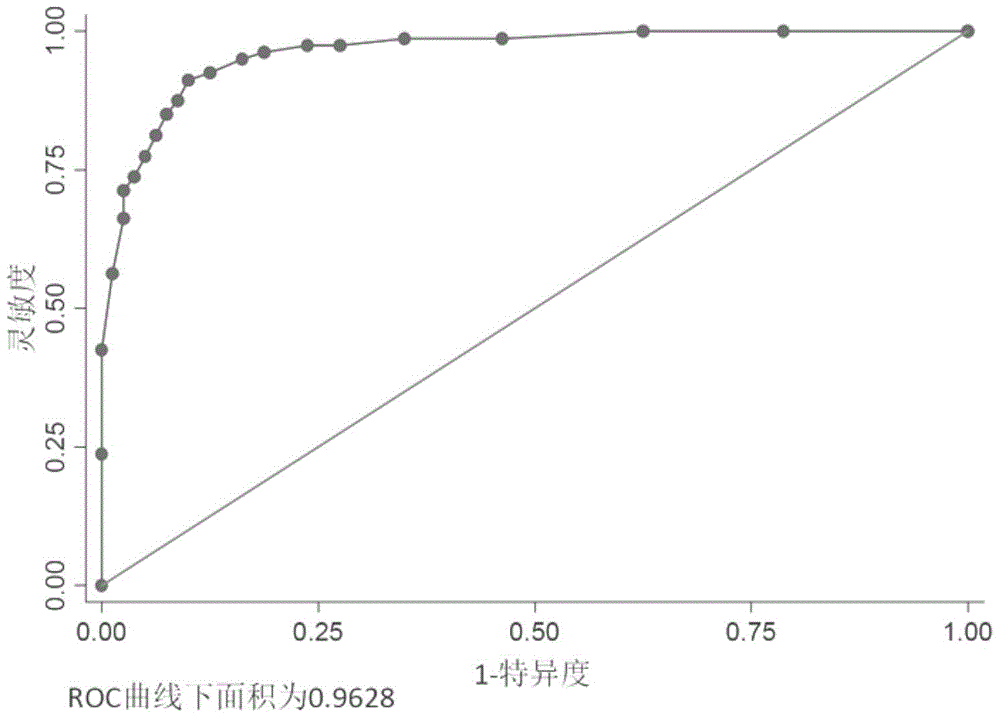
[0099] Primers (Table 1) were designed for quantitative Real-time PCR detection of each miRNAs in the serum of 80 healthy controls and 80 patients with fetal growth restriction.
[0100] (1) Preparation of cDNA samples: a) Take 100 μl of serum; b) Add 60 μl of lysate, vortex for 5 s, and let stand at room temperature for 3 min; c) Add 20 μl of protein solution, vortex for 5 s, stand at room temperature for 1 min, centrifuge at 11,000 g for 3 min; d ) Take the supernatant to a new 2ml collection tube, add 270μl isopropanol, and vortex for 5s; e) Put the microRNA spin column in the collection tube, add the liquid in step d to the spin column, let stand at room temperature for 2min, and centrifuge at 11000g 30s, pour off the liquid in the collection tube, put the spin column back into the collection tube; f) Add 100μl eluent 1 to the microRNA spin column, centrifuge at 11000g for 30s, pour off the liquid in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com