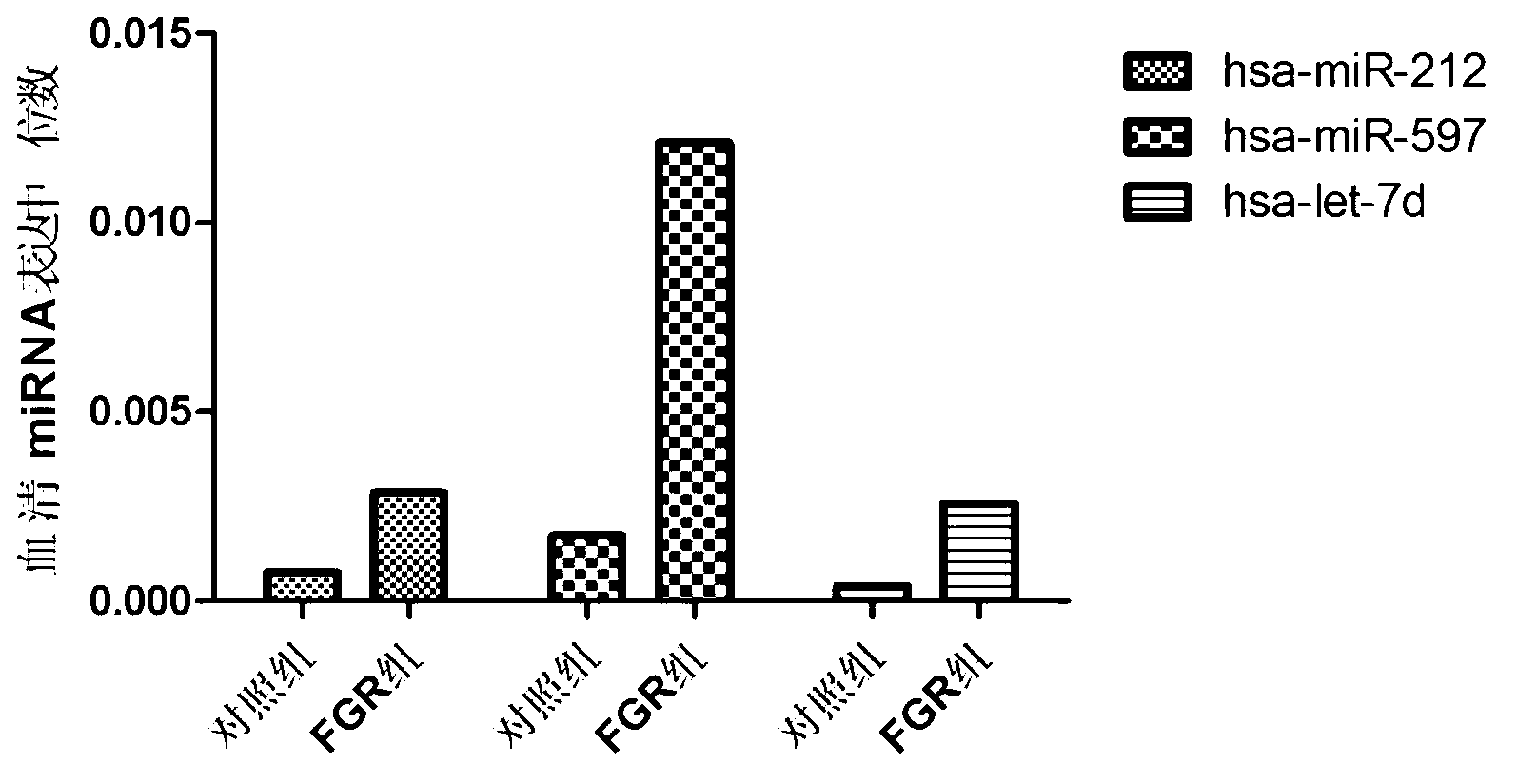Patents
Literature
35results about How to "Avoid invasive diagnostics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Seminal plasma microRNA markers associated with human non-obstructive azoospermia and their application
ActiveCN102296112AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationDynamic monitoringGenetic engineering
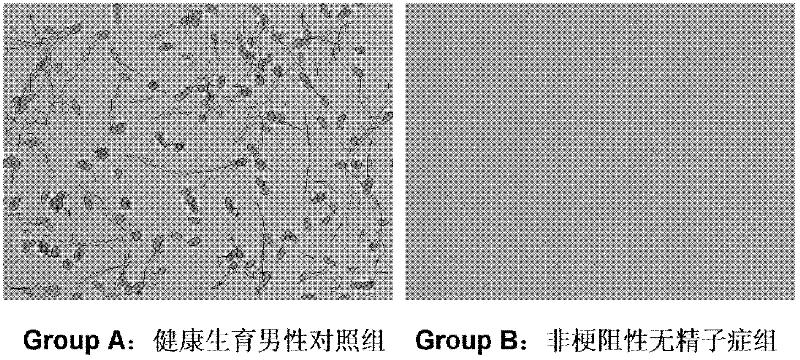
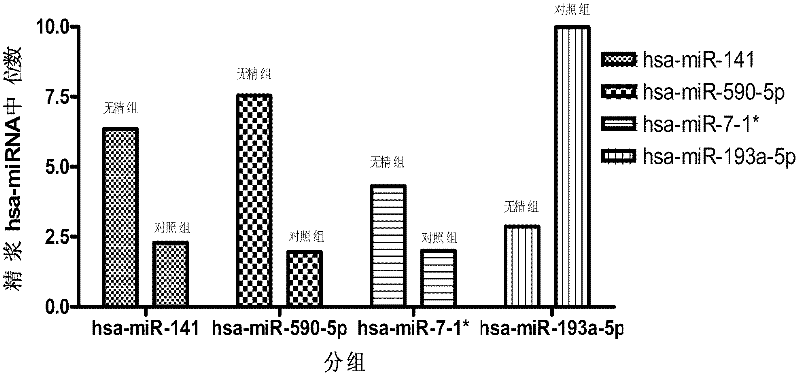
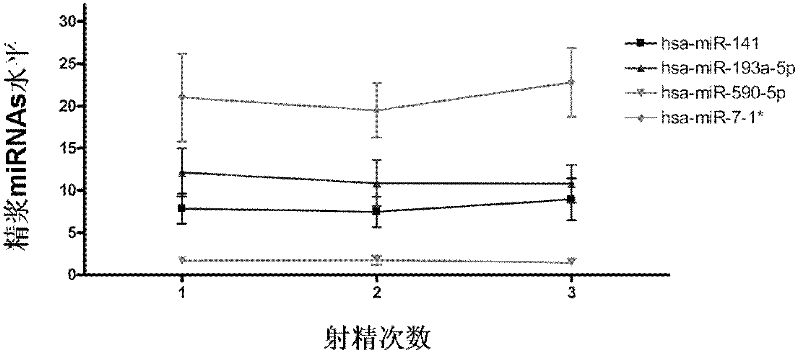
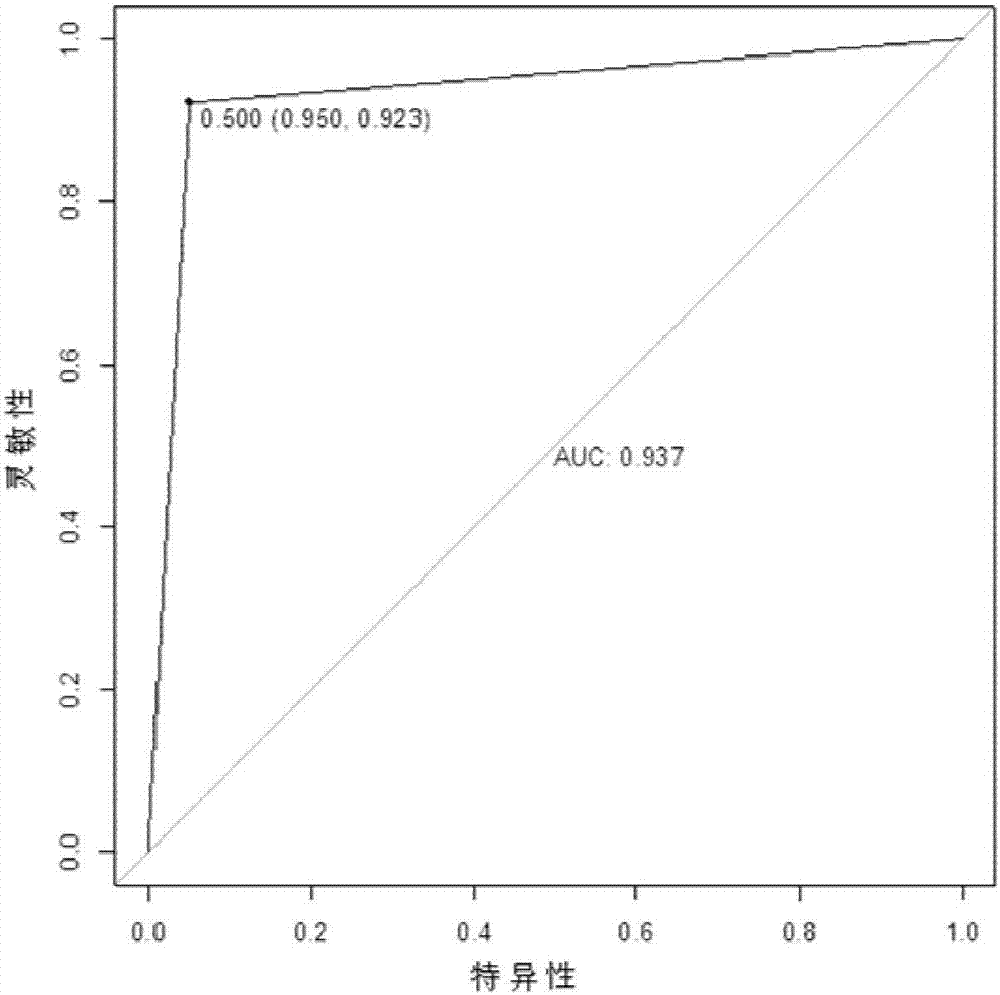
The invention which belongs to the medical field of genetic engineering and reproduction discloses a seminal plasma miRNA marker associated with human non-obstructive azoospermia and an application thereof. The maker is selected from several of hsa-miR-141, hsa-miR-193a-5p, hsa-miR-590-5p and hsa-miR-7-1*. The maker which has specificities and sensitivities to the non-obstructive azoospermia can be used for the preparation of a reagent for non-obstructive azoospermia diagnosis or monitoring, so invasive diagnosis is avoided, repeated detection is realized, and the dynamic monitoring of the obstacle degree of sperm generation can be easily achieved.
Owner:NANJING MEDICAL UNIV
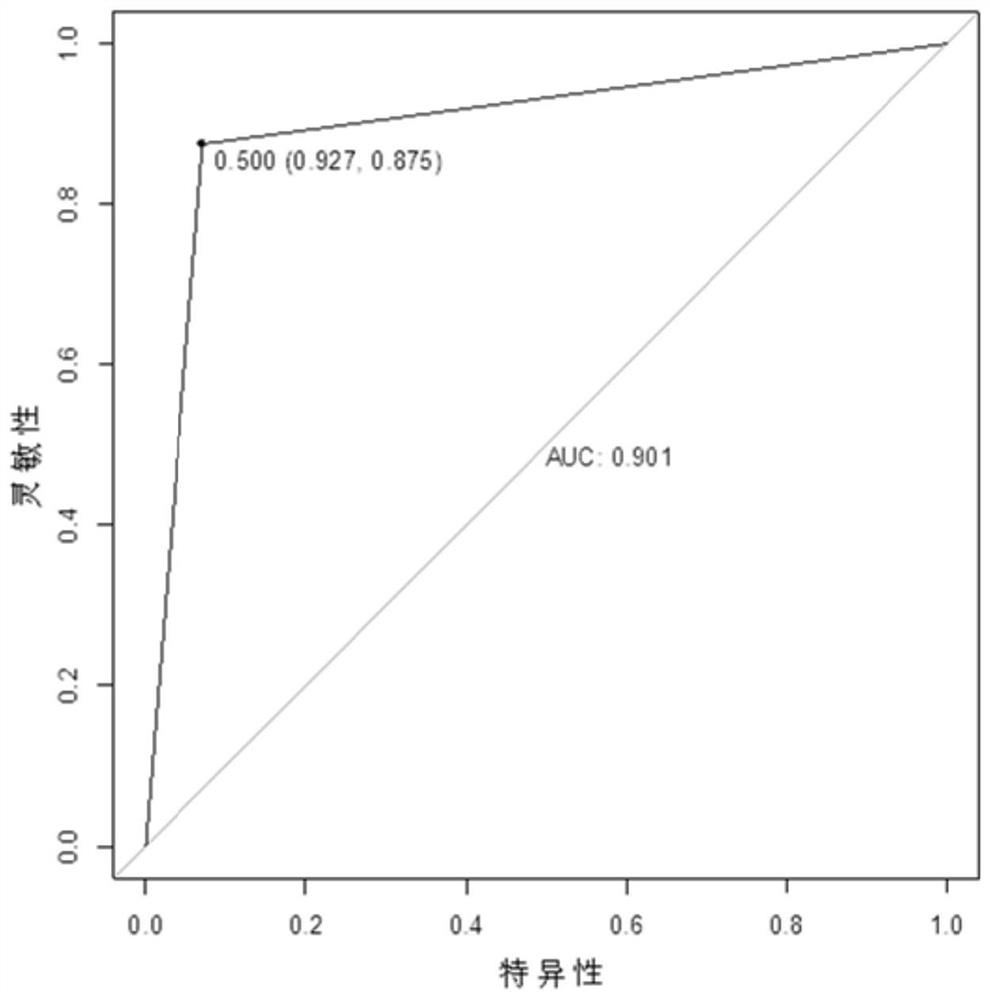
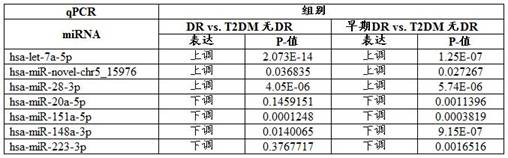
Serum/plasma miRNA marker related to type 2 diabetic retinopathy and use thereof
ActiveCN107385035AGood technical effectIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiabetes retinopathyDiabetic retina
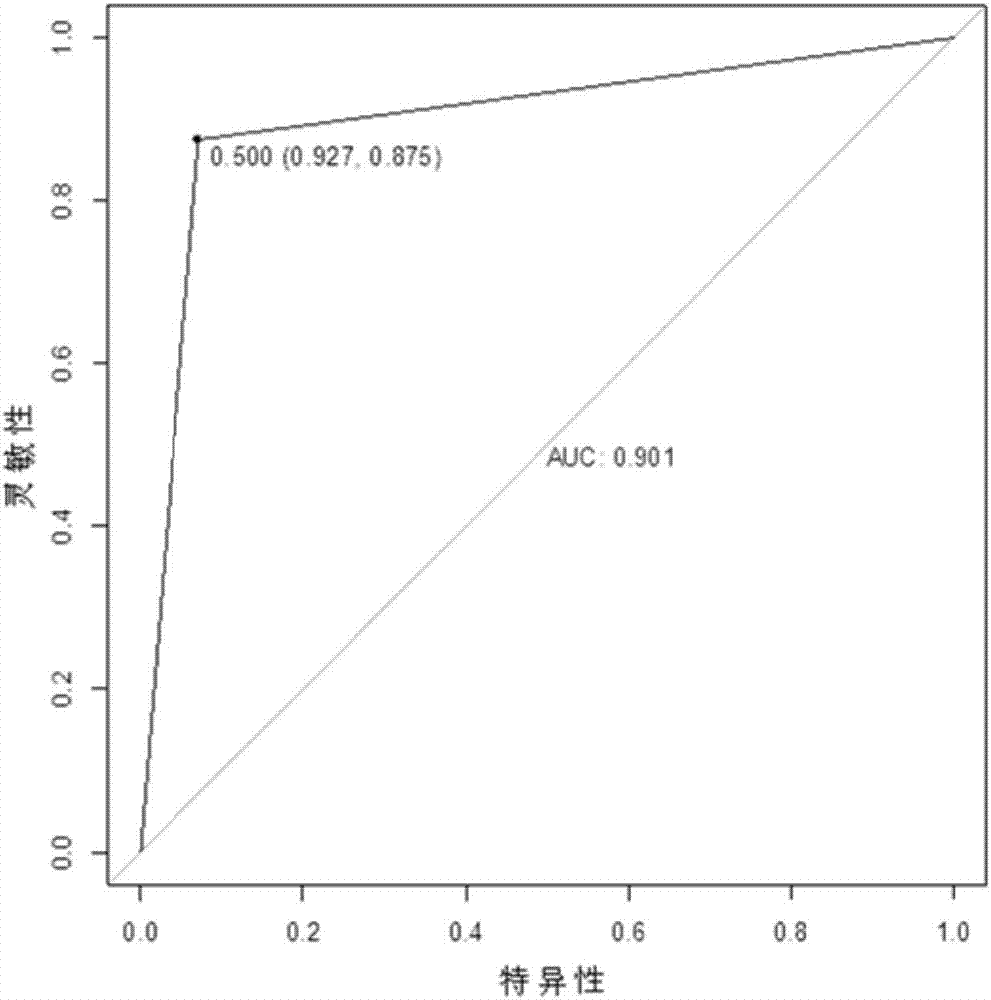
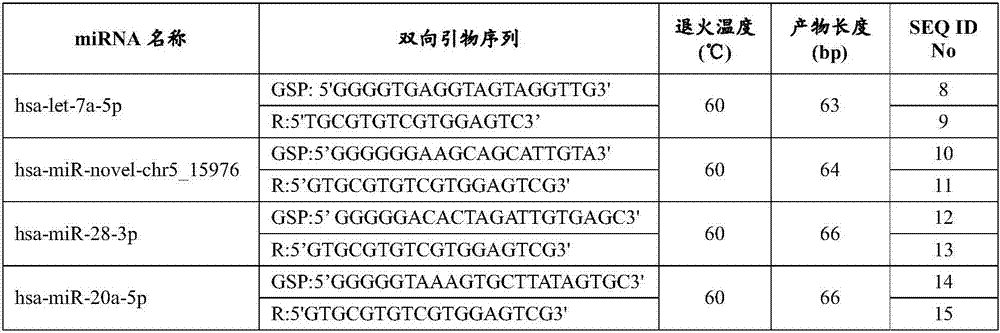
The present invention provides a serum / plasma miRNA marker related to type 2 diabetic retinopathy and use thereof. The serum / plasma miRNA marker related to the type 2 diabetic retinopathy is one or a combination of two of hsa-let-7a-5p, hsa-miR-novel-chr5_15976, hsa-miR-28-3p, has-miR-20a-5p, has-miR-151a-5p, has-miR-148a-3p and has-miR-223s-3p. The serum / plasma miRNA marker related to the type 2 diabetic retinopathy is stable and can be used to early assist to diagnose whether a diabetic patient is combined with DR, and the disease progression of the diabetic retinopathy patient can be dynamically monitored, and the serum / plasma miRNA marker can be used to prepare an early diagnosis product of type 2 diabetes and the diabetic retinopathy.
Owner:SHENZHEN UNIV +1
Plasma micro-ribonucleic acid (miRNA) marker related with human Hirschsprung's disease and application of miRNA marker
ActiveCN102358900AEasy diagnosisHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseDynamic monitoring
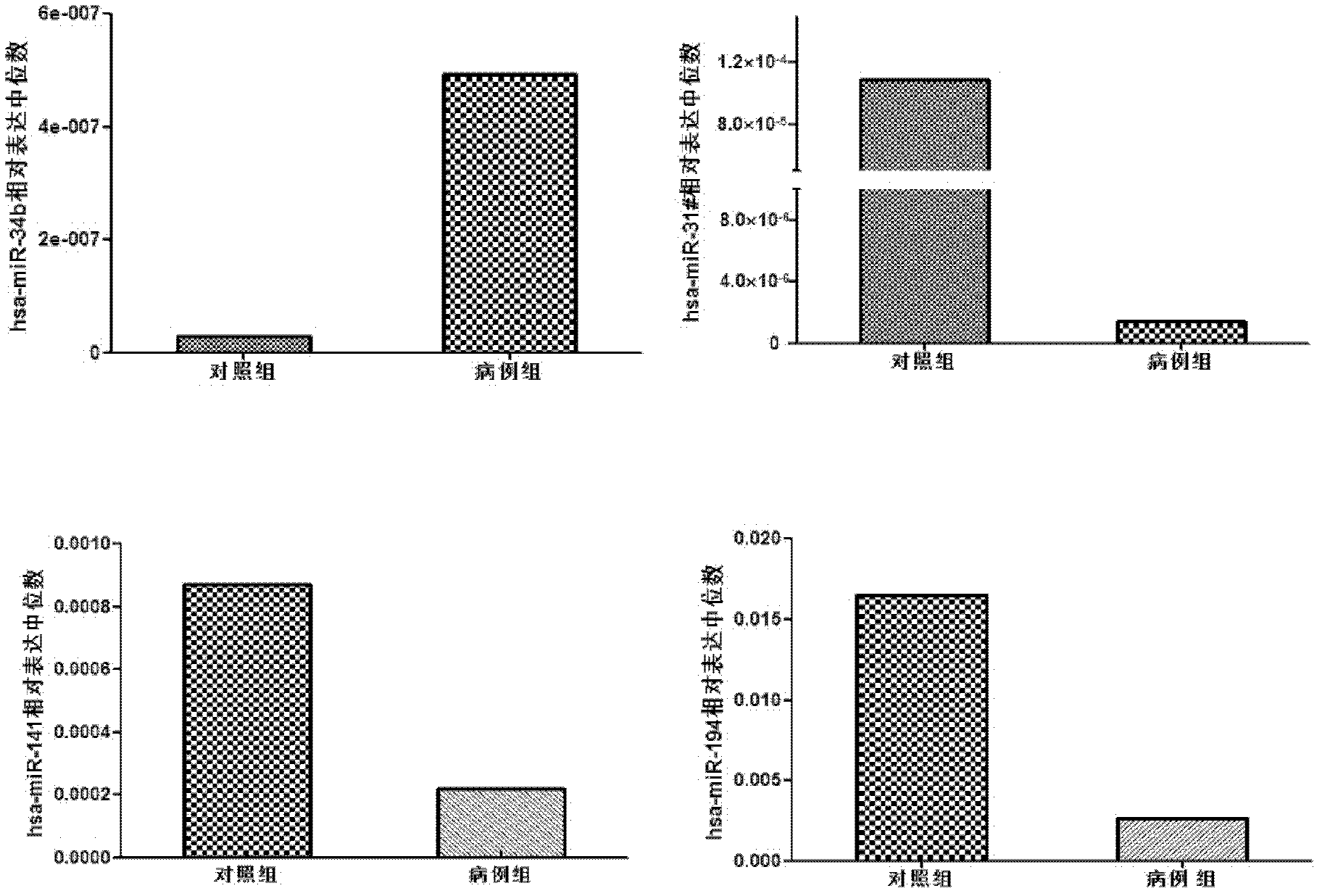
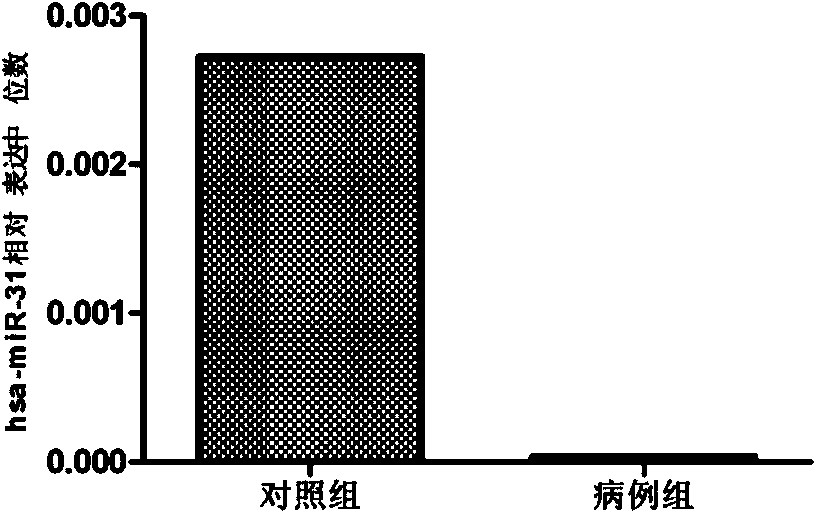
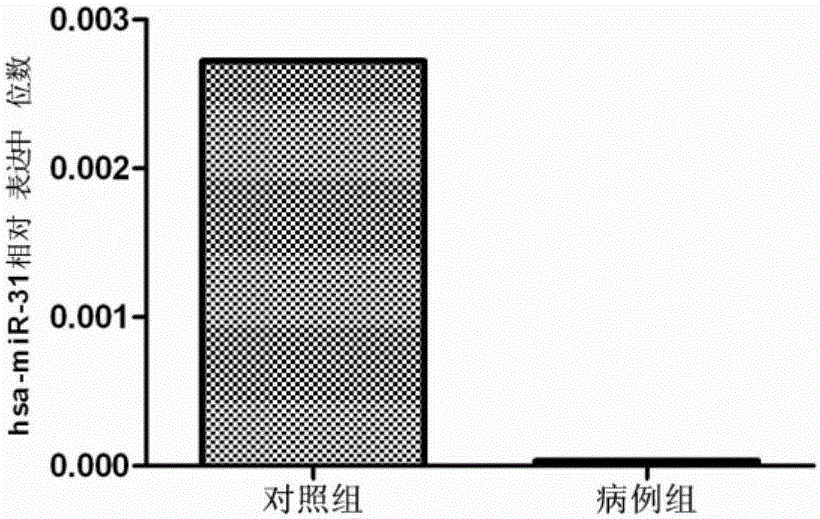
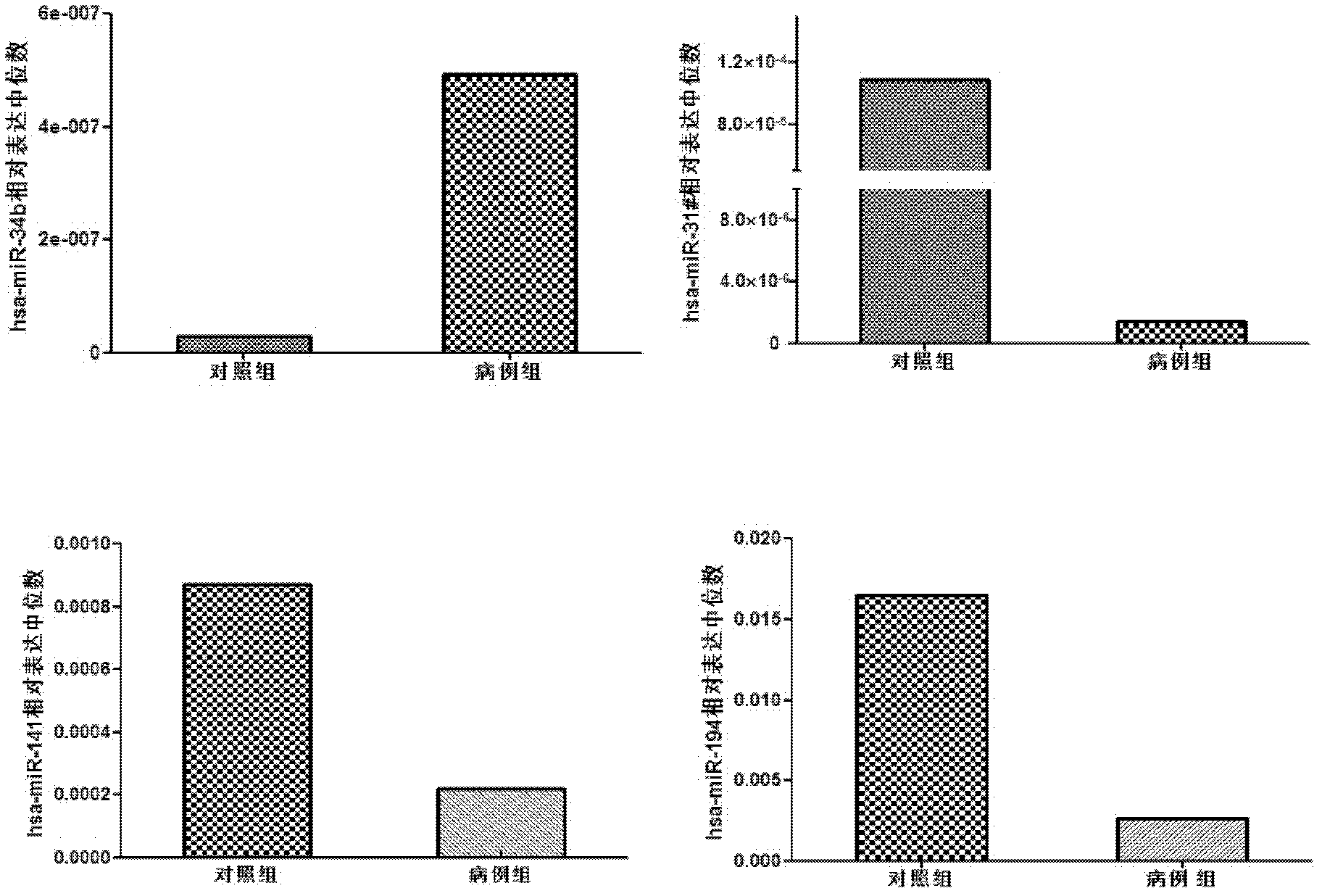
The invention belongs to the fields of gene engineering and clinical medicine, and discloses a plasma micro-ribonucleic acid (miRNA) marker related with a human Hirschsprung's disease and application of the miRNA marker. The marker is selected from multiple kinds of hsa-miR-34b, hsa-miR-31*, hsa-miR-141 and hsa-miR-194. The marker has specificity and sensitivity on the Hirschsprung's disease, canbe used for preparing a reagent for diagnosing or monitoring the Hirschsprung's disease, can avoid invasive diagnosis, can be used for screening and diagnosis at the early stage, can be repeatedly detected, and is easy in dynamic monitoring.
Owner:NANJING MEDICAL UNIV
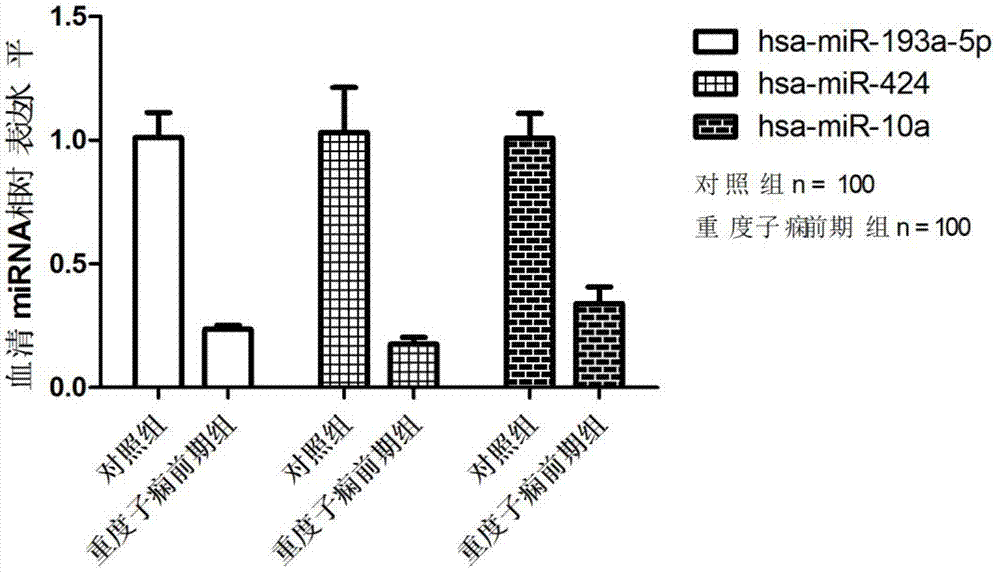
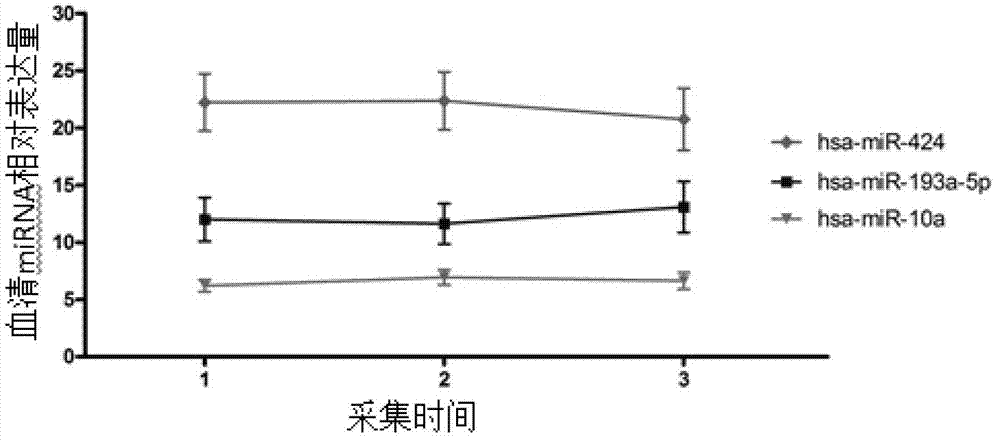
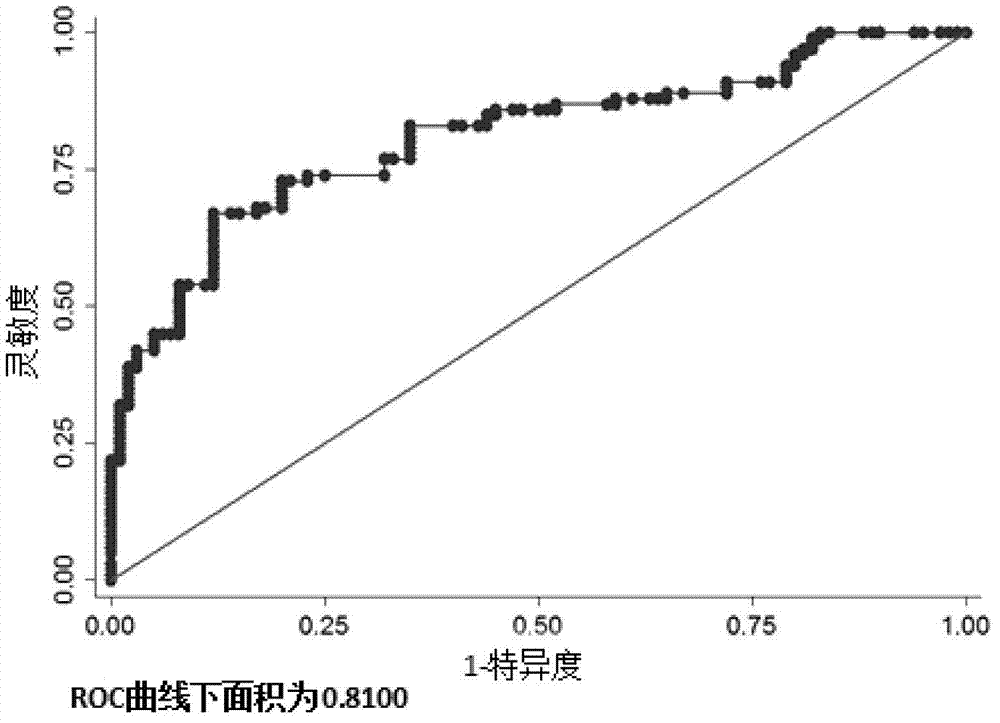
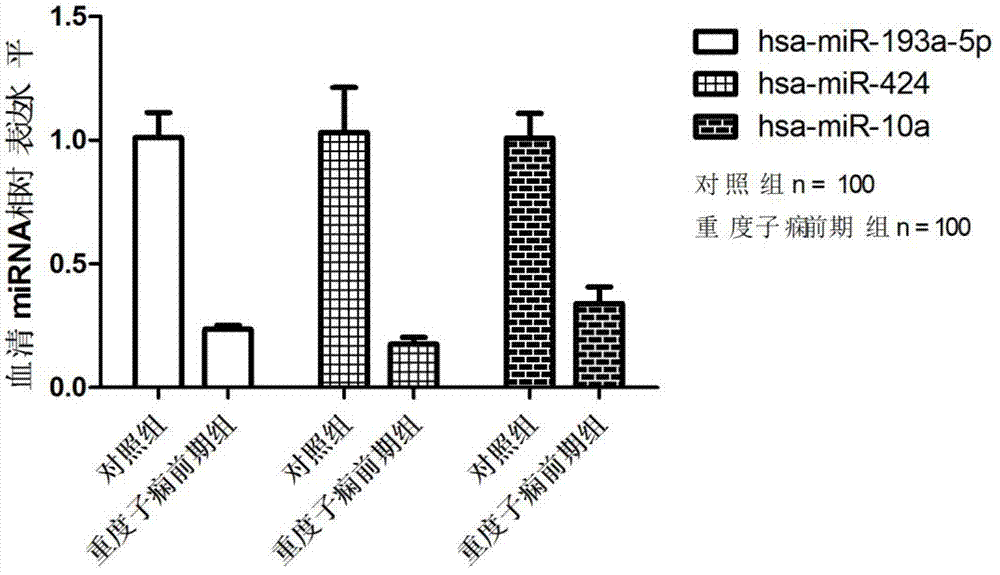
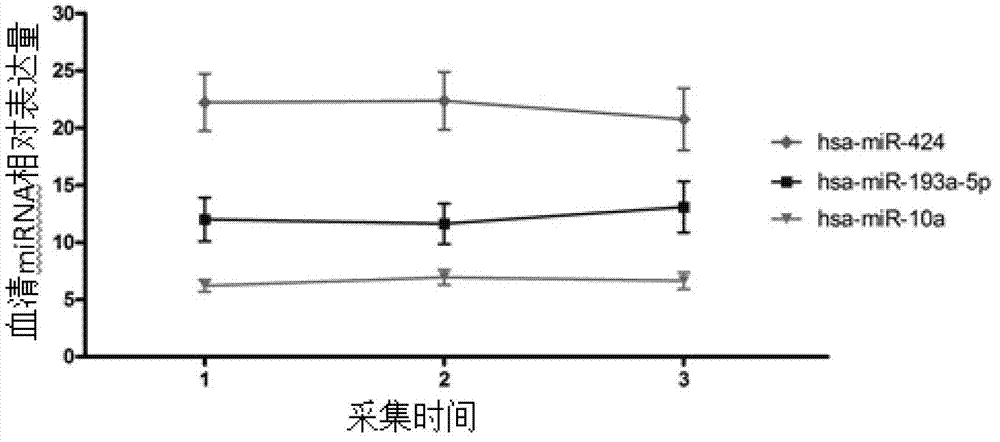
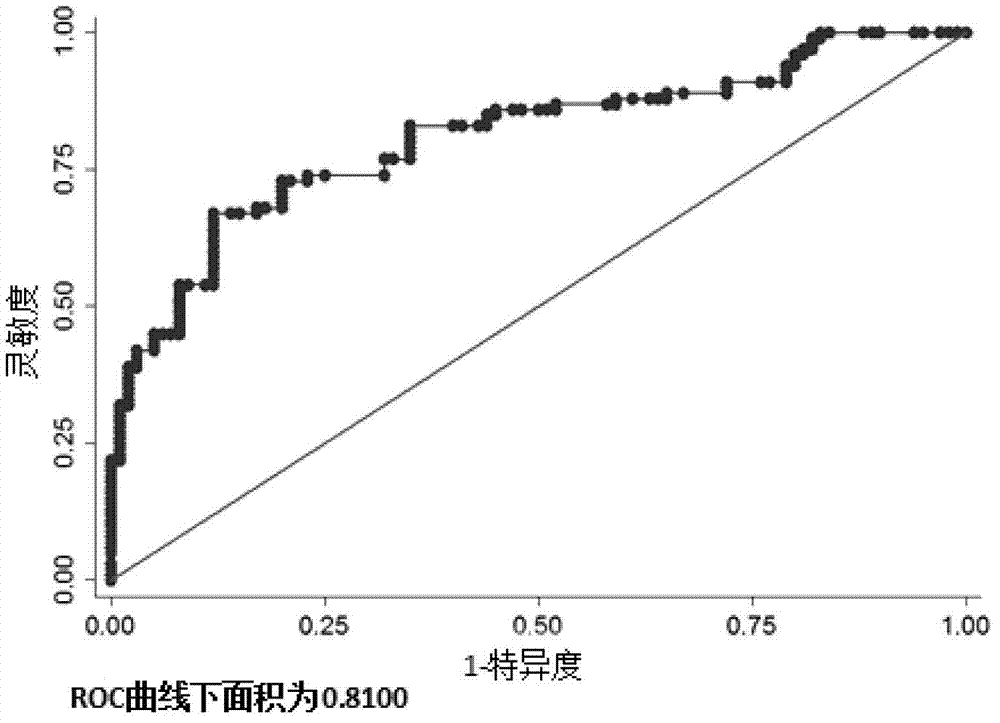
Related serum microribonucleic acid marker for human severe preeclampsia and application of marker
ActiveCN103205430AEasy to monitorHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGenetic engineeringBlood serum
The invention belongs to the fields of genetic engineering and clinical medicine, and discloses a related serum microribonucleic acid marker for human severe preeclampsia and application of the marker. The marker is selected from more of hsa-miR-193a-5p, hsa-miR-424 and hsa-miR-10a. The marker has the specificity and sensitivity on a sufferer with severe preeclampsia, and can be used for preparing a diagnosis or monitoring kit for severe preeclampsia.
Owner:夏彦恺
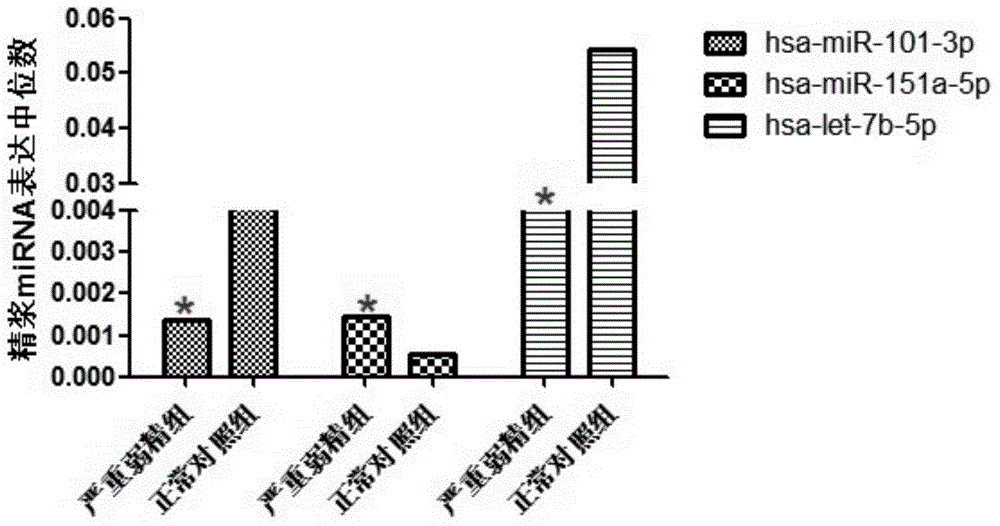
Mitochondria-related seminal plasma miRNAs taken as mankind severe asthenospermia markers, and applications thereof
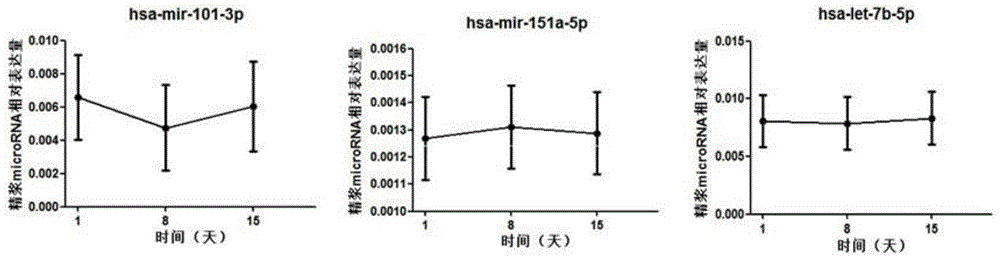
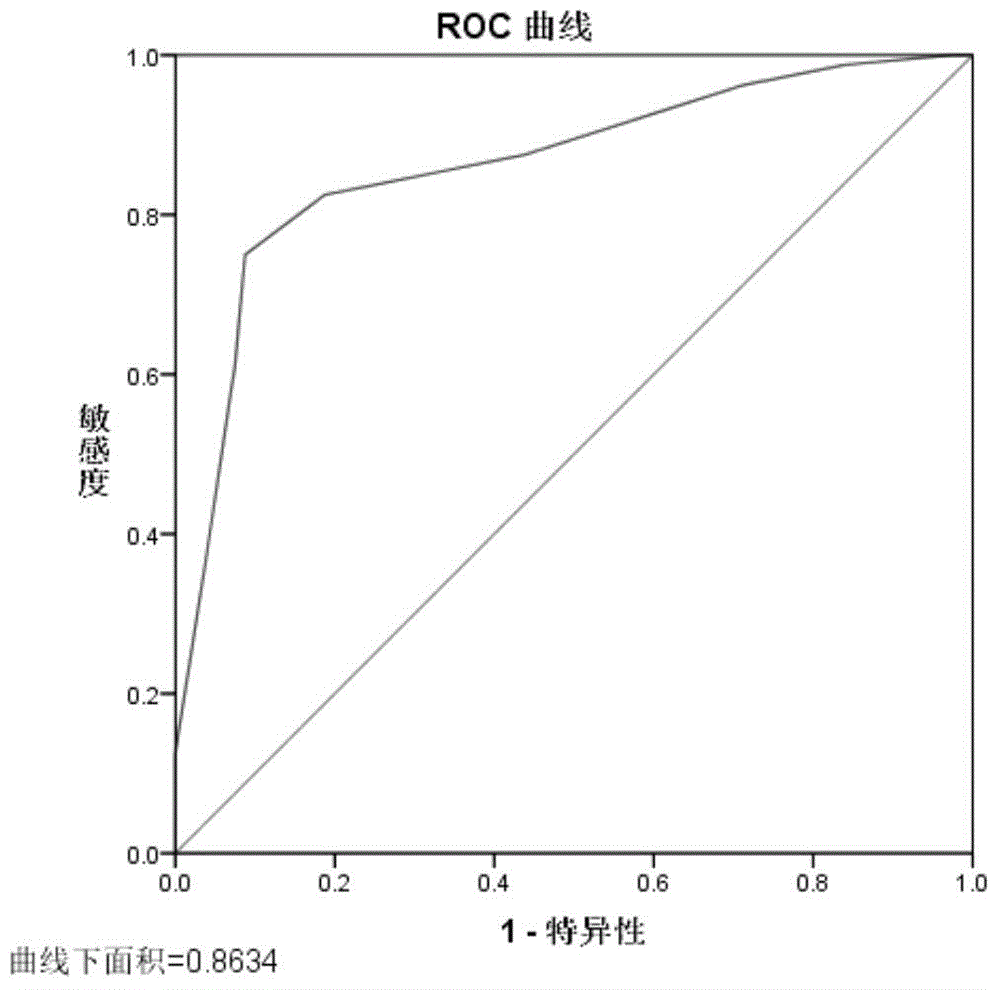
ActiveCN105039530AConvenient for clinical operationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMir 101 3pNormal fertility
The invention belongs to the field of gene engineering and reproductive medicine, and discloses mitochondria-related seminal plasma miRNAs taken as mankind severe asthenospermia markers, and applications thereof. The markers are mitochondria-related miRNAs selected from hsa-miR-101-3p, hsa-miR-151a-5p, and hsa-let-7b-5p. The markers can be used for separating patients with severe asthenospermia from a control group with normal fertility, and can be used for preparing human severe asthenospermia diagnosis or monitor kits.
Owner:NANJING MEDICAL UNIV
Biomarker for abnormal invasion of trophoblast cells and application thereof
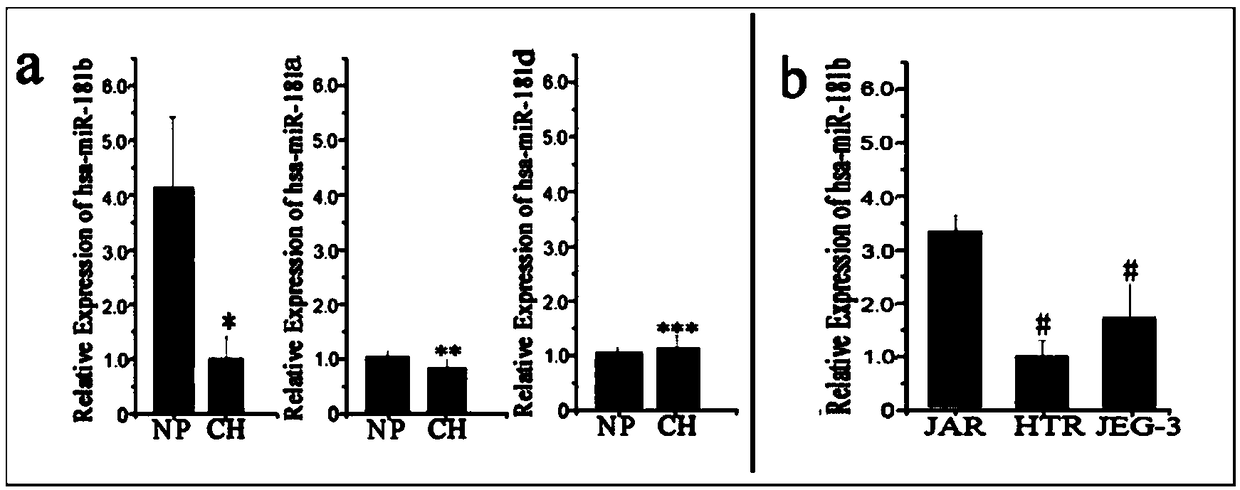
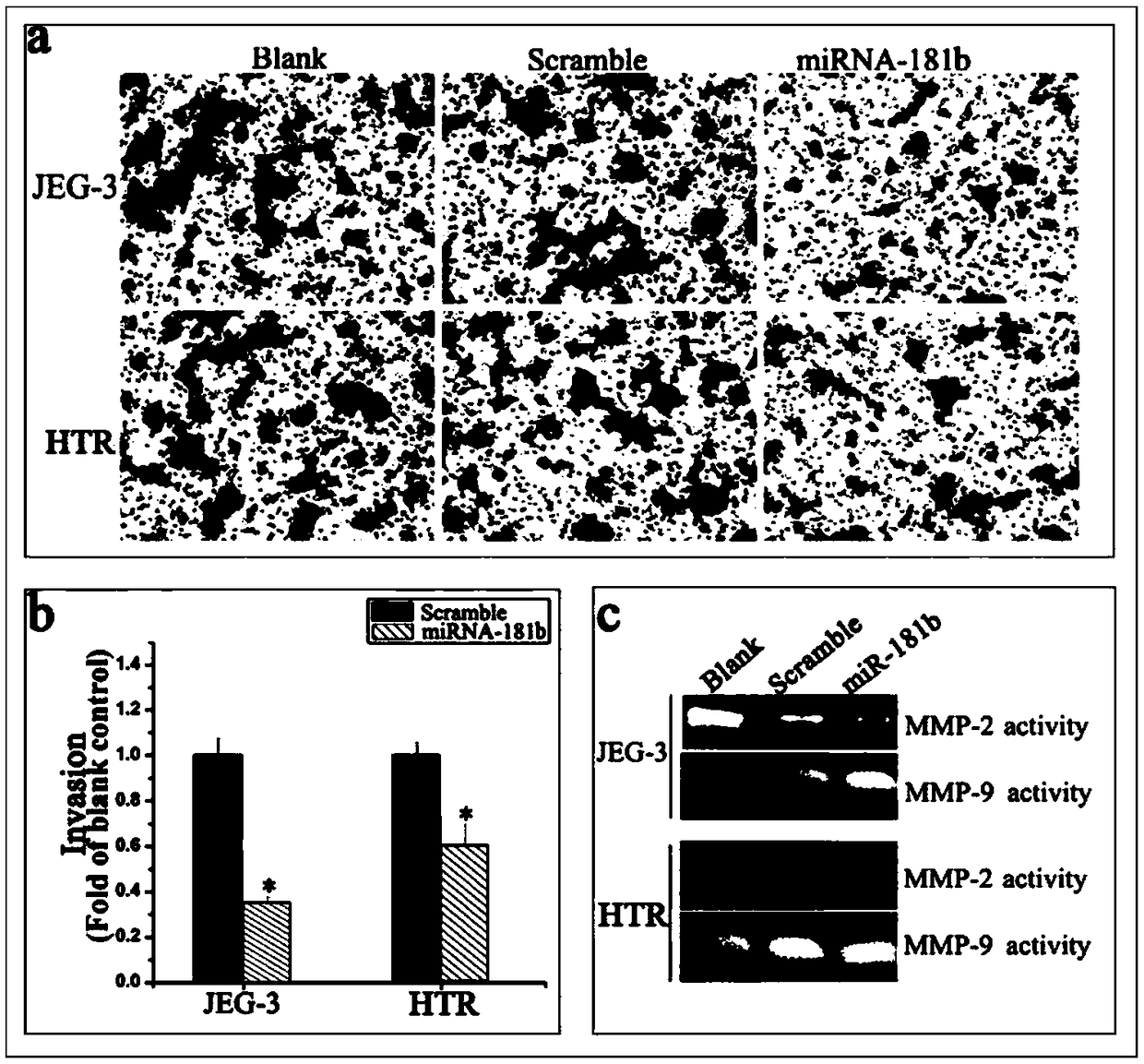
ActiveCN108949973AEasy to detectQuantitatively accurateMicrobiological testing/measurementDiseasePregnancy
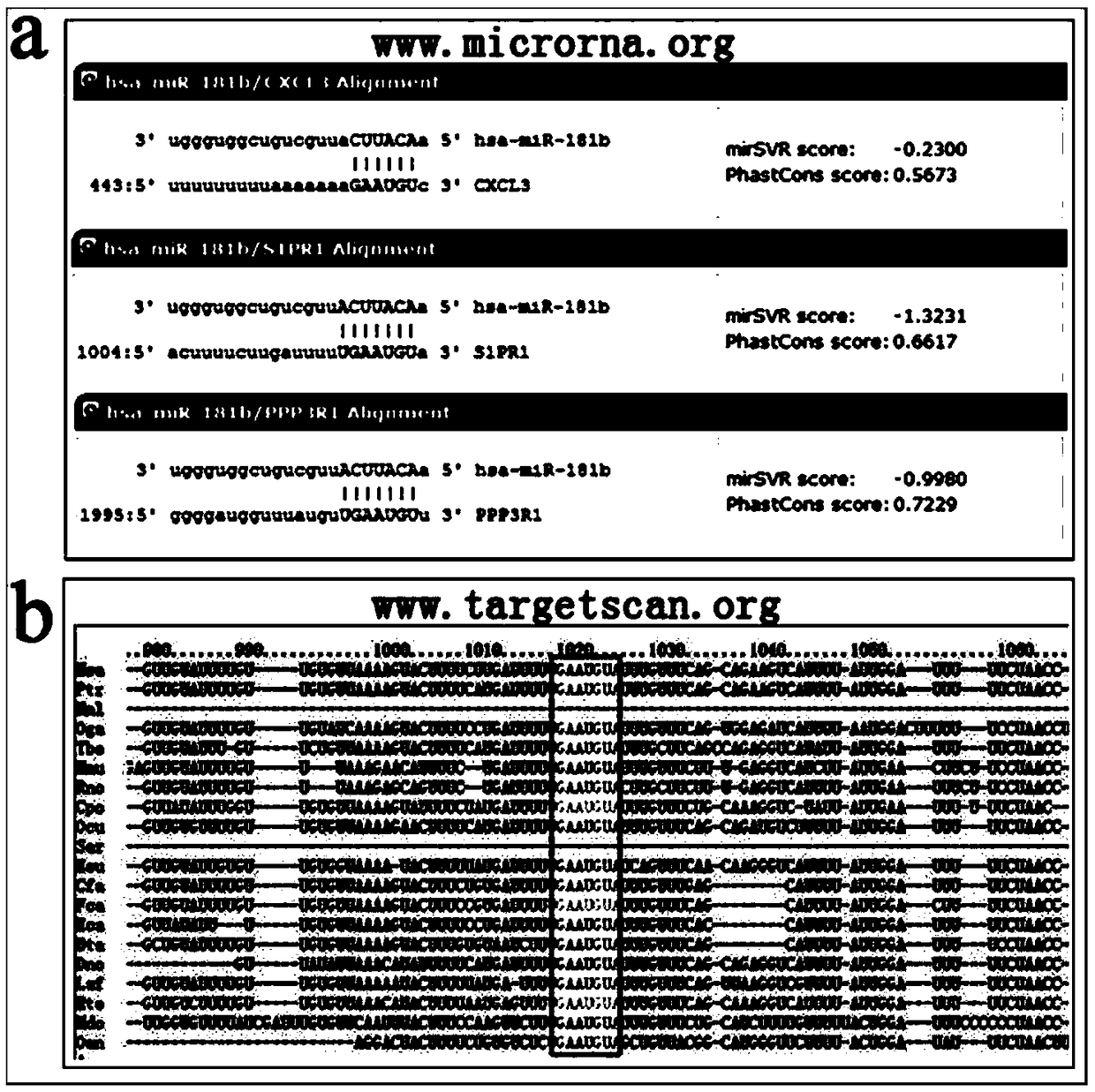
The invention relates to a biomarker for abnormal invasion of trophoblast cells and application thereof. It is proved that miR-181b is generally and differentially expressed in pregnancy-related disease tissue with abnormal trophoblast invasion, and S1PR1 is a direct effect target gene of miR-181b; and the discovery of the influence of a miR-181b-S1PR1 pathway on the molecular mechanism of the invasion ability of trophoblast cells provides new clues and potential intervention targets for the prevention and treatment of pregnancy-related diseases caused by abnormal trophoblast invasion.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
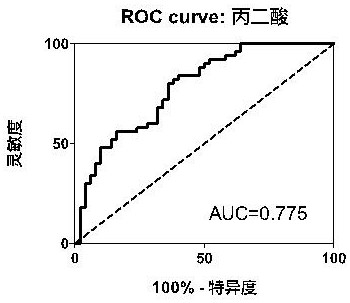
Plasma metabolization micromolecule marker related to human non-small-cell lung cancer and application of plasma metabolization micromolecule marker
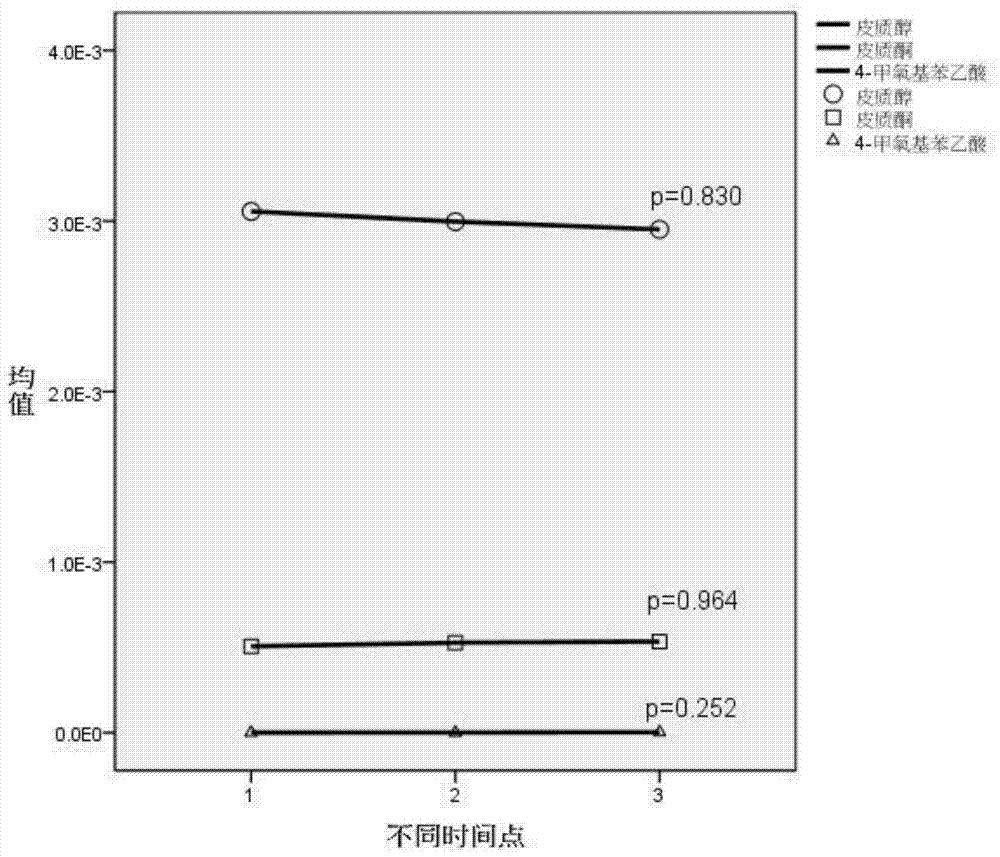
InactiveCN105203683ARepair inhibitionIncrease contractilityComponent separationMetaboliteConfidence interval
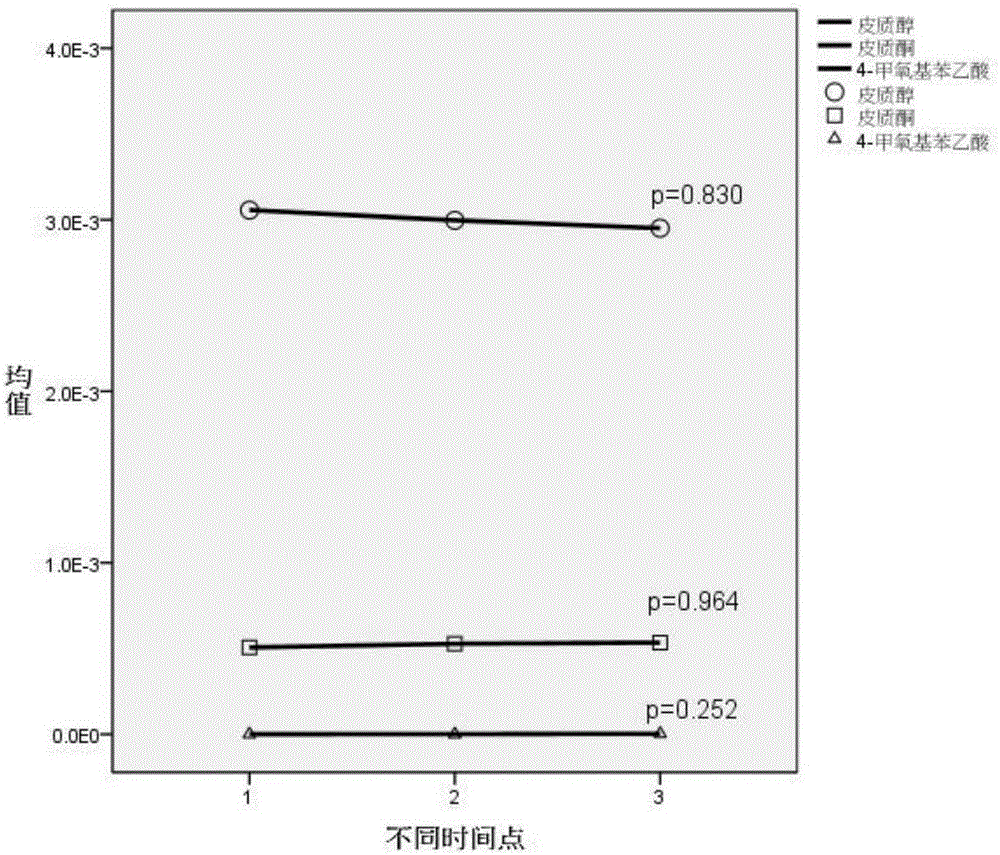
The invention belongs to the field of analytical chemistry and clinical medicine and relates to a plasma metabolization micromolecule marker related to the human non-small-cell lung cancer and an application of the plasma metabolization micromolecule marker. The plasma metabolization micromolecule marker related to the human non-small-cell lung cancer is one or more of cortisol, corticosterone and 4-methoxyphenylacetic acid. The plasma metabolization micromolecule marker is prepared from cortisol, corticosterone and 4-methoxyphenylacetic acid. The content range (95% confidence interval) of cortisol is 0.00018-0.00067, the content range (95% confidence interval) of corticosterone is 0.000029-0.00010, the content range (95% confidence interval) of 4-methoxyphenylacetic acid is 0.000015-0.000022, and metabolite can prompt occurrence of tumors within the ranges. The horizontal range, corresponding to a normal group, of cortisol is 0.0030-0.0037, the horizontal range, corresponding to the normal group, of corticosterone is 0.00044-0.00056, and the horizontal range, corresponding to the normal group, of 4-methoxyphenylacetic acid is 7.39 E-07-2.09 E-06. The plasma metabolization micromolecule marker is a novel biomarker, compared with a traditional protein biomarker, the relevance between the marker and the disease outcome is higher, and the plasma metabolization micromolecule marker is stable, minimally invasive, easy to detect and accurate in quantitation.
Owner:JIANGSU PROVINCE HOSPITAL
Plasma miRNA (micro ribonucleic acid) marker associated with common type congenital intestinal canal total colonic aganglionosis and application thereof
ActiveCN103805603AEasy to monitorHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDynamic monitoringBlood plasma
The invention belongs to the fields of genetic engineering and clinical medicines, and discloses a plasma miRNA (micro ribonucleic acid) marker associated with common type congenital intestinal canal total colonic aganglionosis and an application thereof. The marker is selected from more of hsa-miR-31, hsa-miR-147 and hsa-miR-206. The marker has specificity and sensitivity to the common type congenital intestinal canal total colonic aganglionosis, can be used for the preparation of reagents for diagnosing or monitoring the common type congenital intestinal canal total colonic aganglionosis, can avoid invasive diagnosis, can be used for screening and diagnosis in an early stage, and can be used for detecting repeatedly and is easy for dynamic monitoring.
Owner:NANJING CHILDRENS HOSPITAL AFFILIATED TO NANJING MEDICAL UNIV
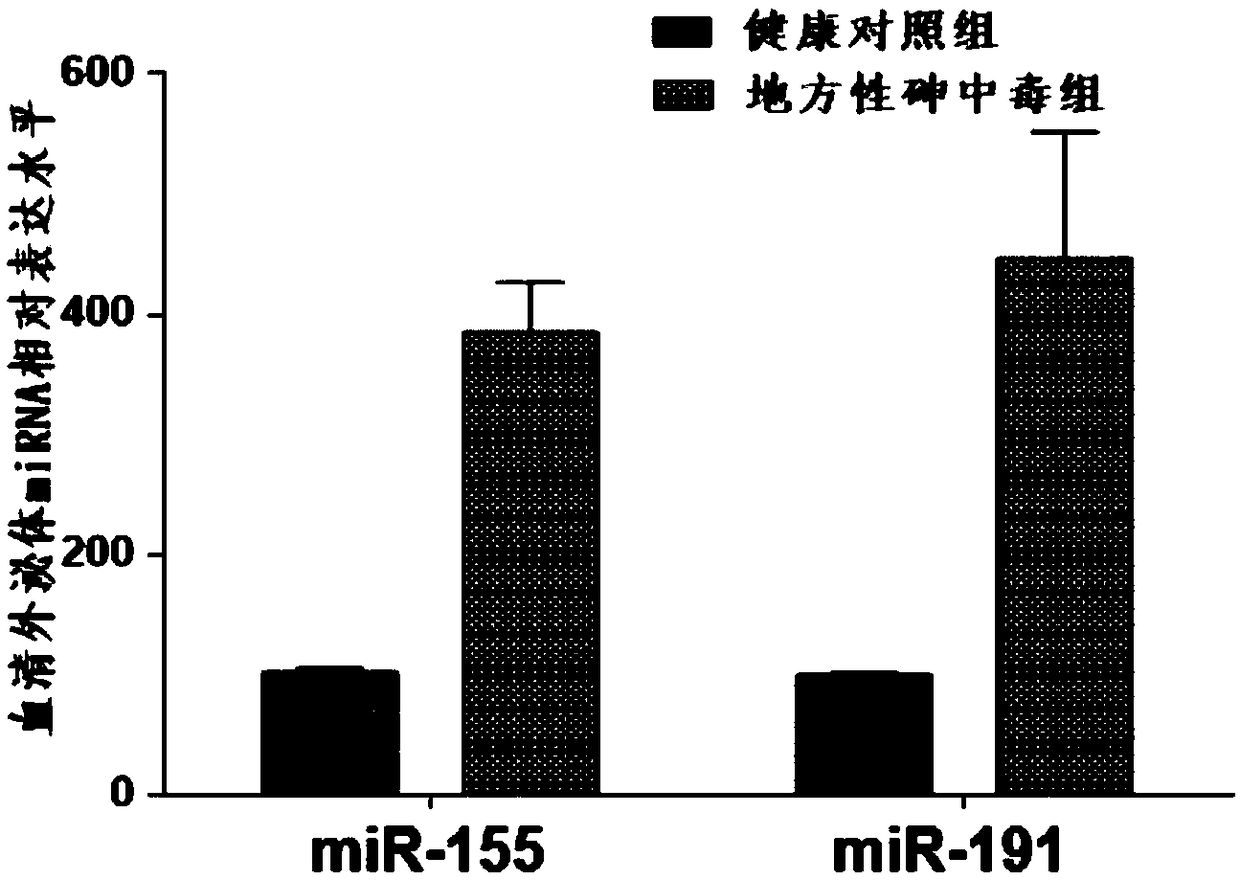
Application of serum exosome miRNAs marker to early diagnosis of endemic arsenism
InactiveCN106435004AEasy extractionReduce the amount of serum drawnMicrobiological testing/measurementDNA/RNA fragmentationSerum igeExosome
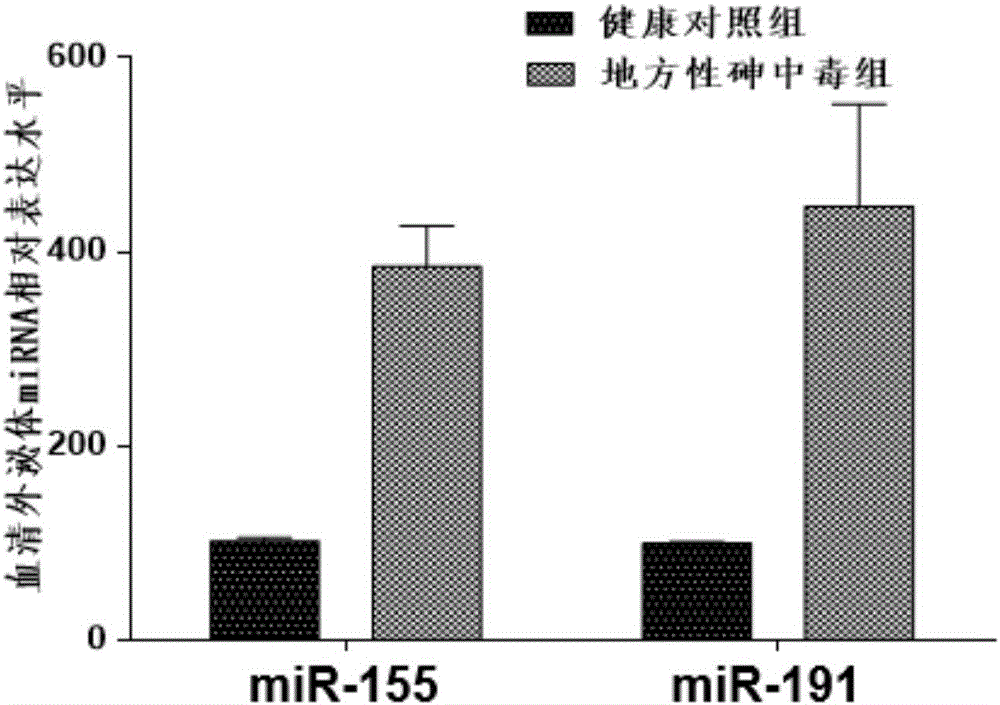
The invention belongs to the field of gene engineering and clinical medicine and particularly relates to an endemic arsenism early diagnosis associated serum exosome miRNAs marker and application thereof. The marker is a combination of has-miR-155 and has-miR-191. The marker and a primer thereof can be used for preparation of a kit for early diagnosis of endemic arsenism.
Owner:NANJING MEDICAL UNIV
ELISA kit for auxiliary diagnosis of esophageal squamous carcinoma
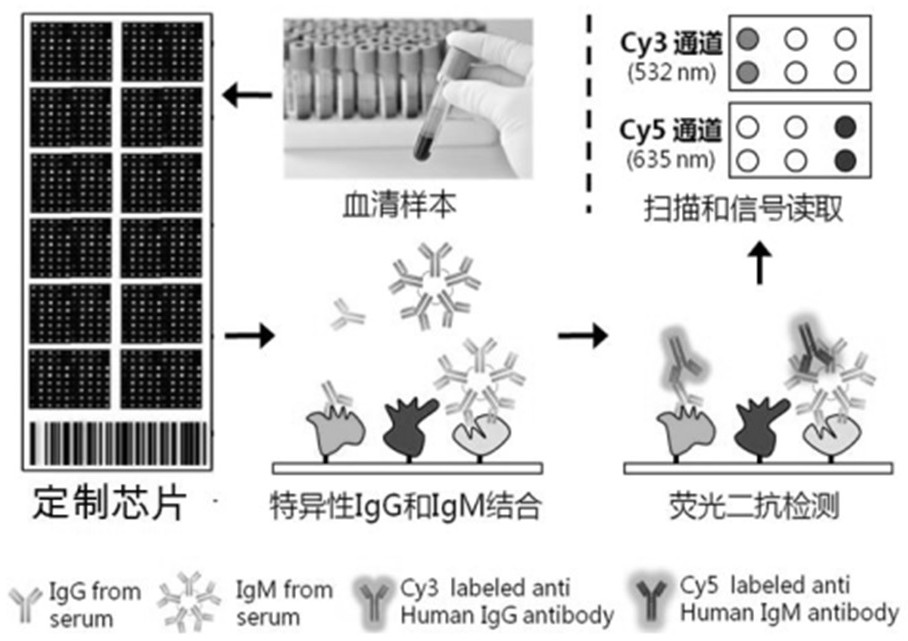
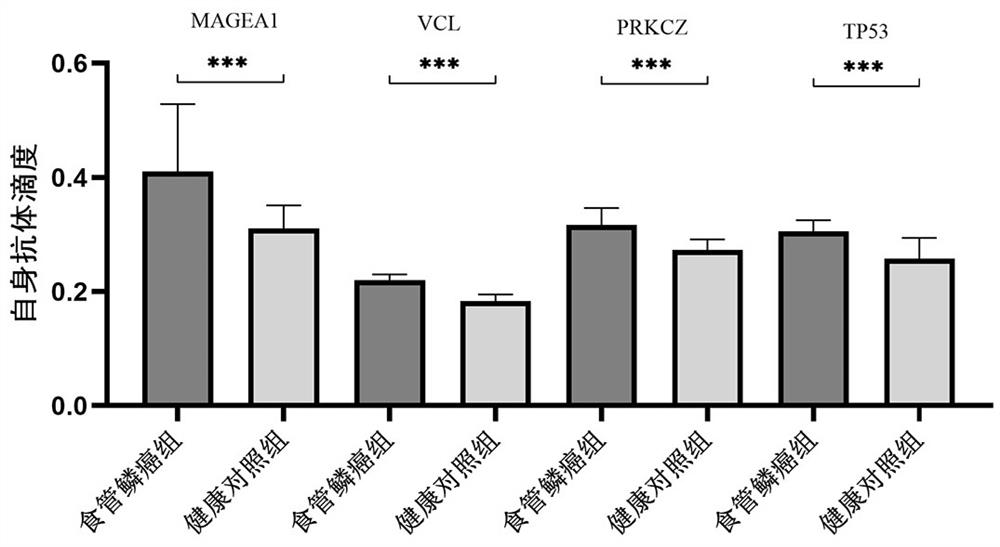
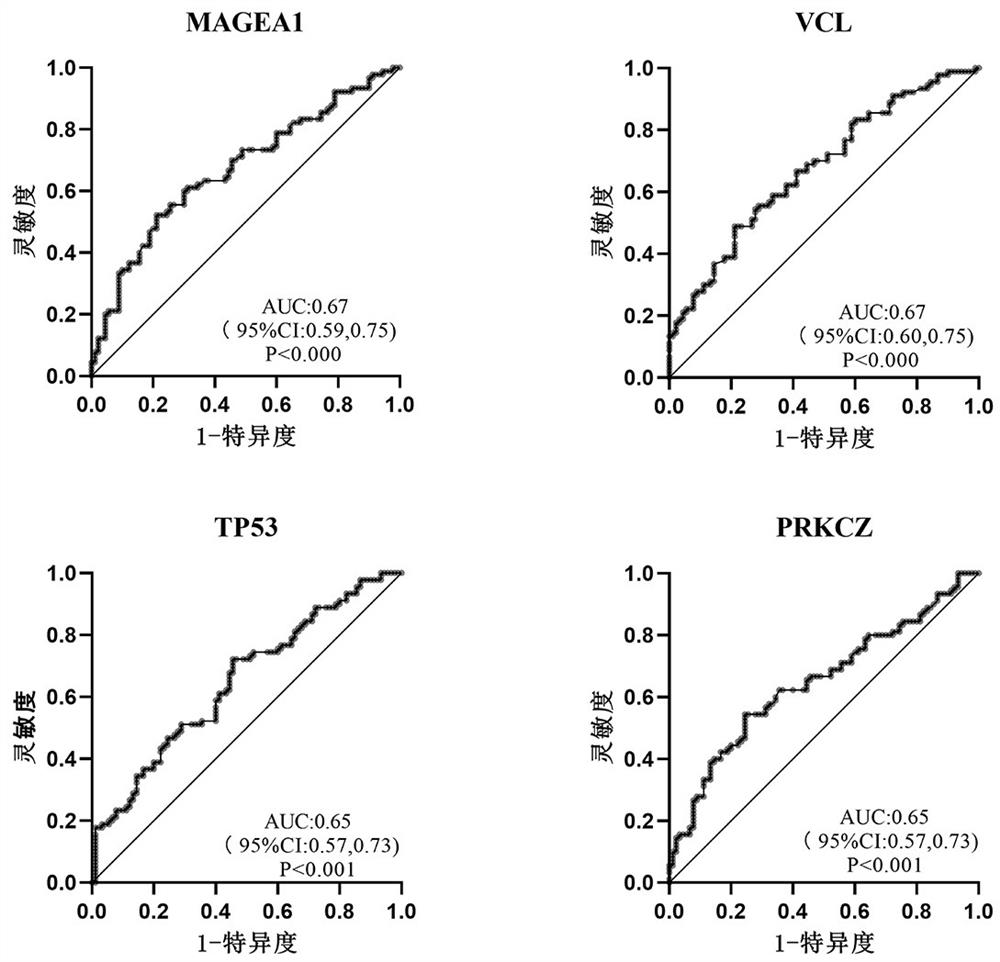
The invention belongs to the technical field of medical biology, and particularly discloses a biomarker for auxiliary diagnosis of esophageal squamous carcinoma. The biomarker for diagnosing esophageal squamous cell carcinoma, provided by the invention, is at least one of autoantibodies of anti-tumor related antigens MAGEA1, VCL, TP53 and PRKCZ. The invention also provides a kit for auxiliary diagnosis of esophageal squamous carcinoma, the kit contains a reagent for detecting the biomarker, and the reagent is used for detecting the biomarker in a sample through enzyme-linked immunosorbent assay, a protein chip, immunoblotting or microfluidic immunoassay. By detecting the expression levels of autoantibodies of anti-tumor related antigens MAGEA1, VCL, TP53 and PRKCZ in human serum, esophageal squamous cell carcinoma patients and normal people can be effectively distinguished, and the kit can be used for auxiliary diagnosis of esophageal squamous cell carcinoma.
Owner:ZHENGZHOU UNIV
Serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof
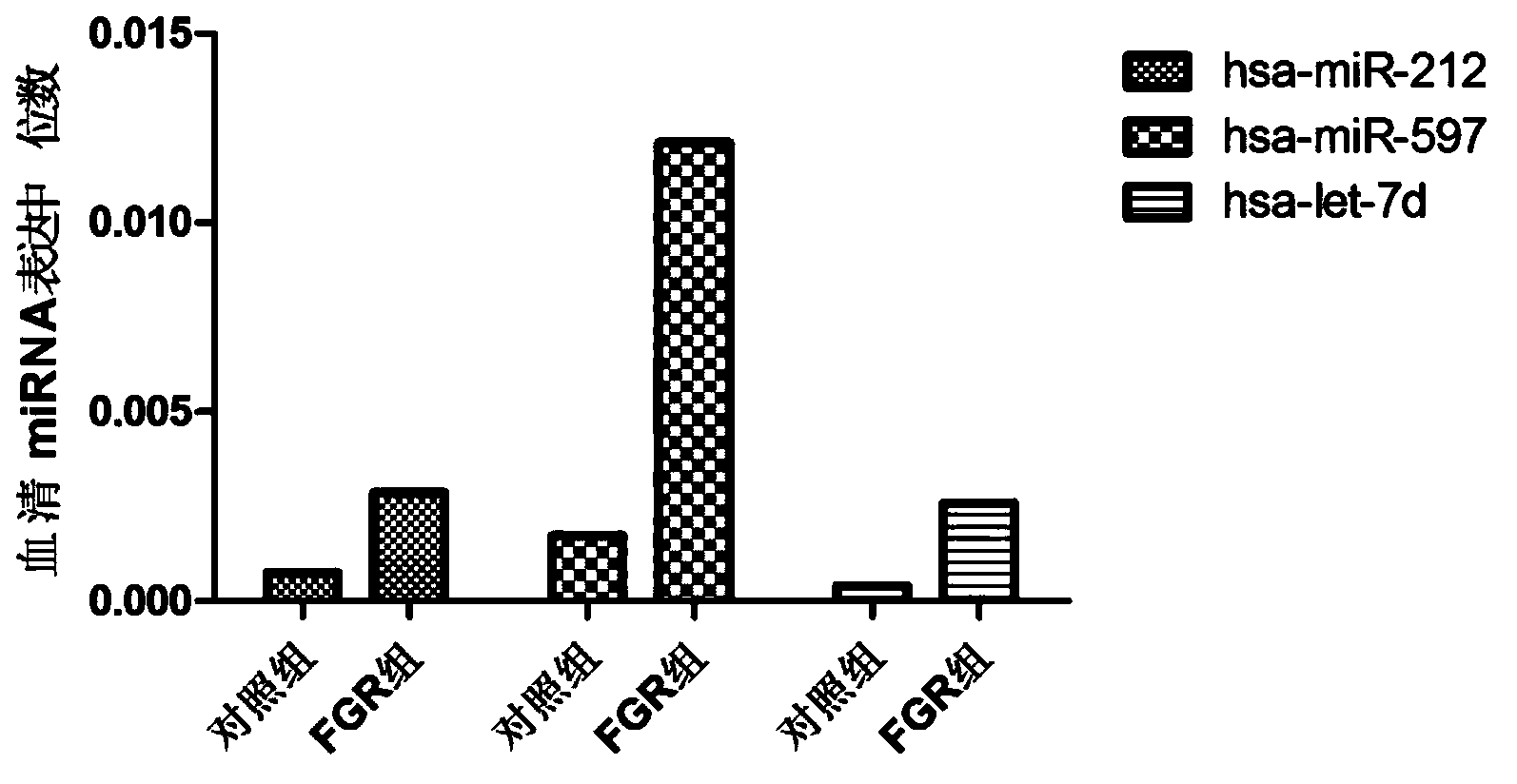
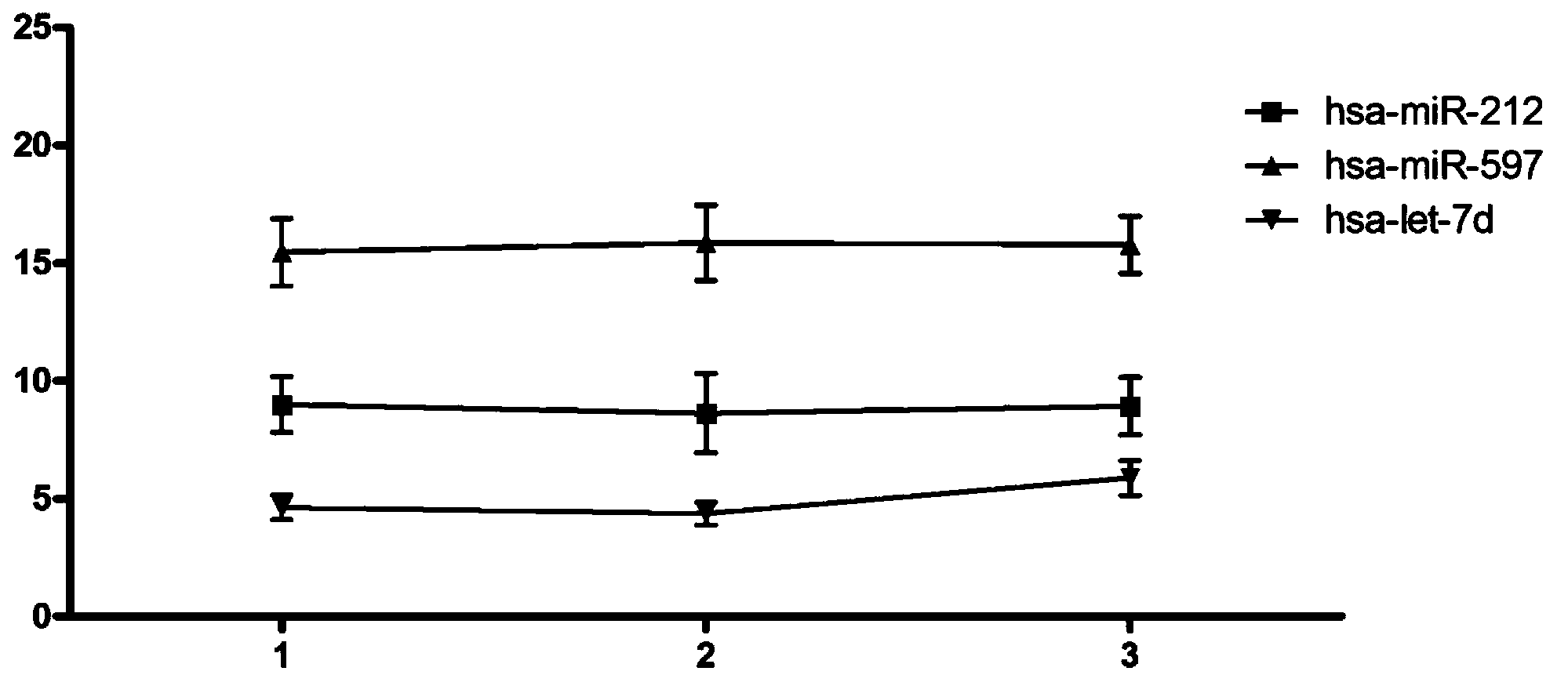
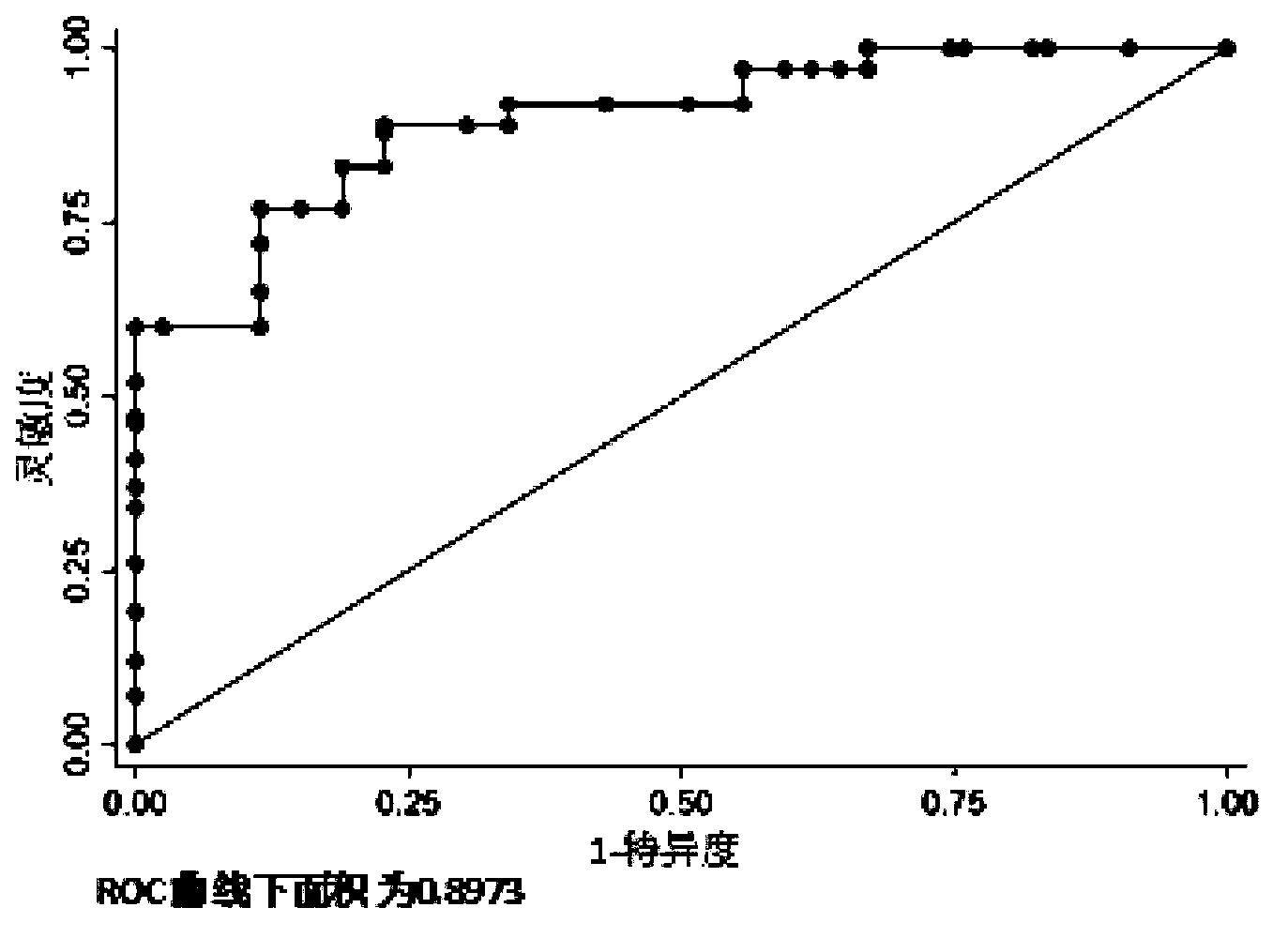
ActiveCN103194542AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationSerum igeFetal growth
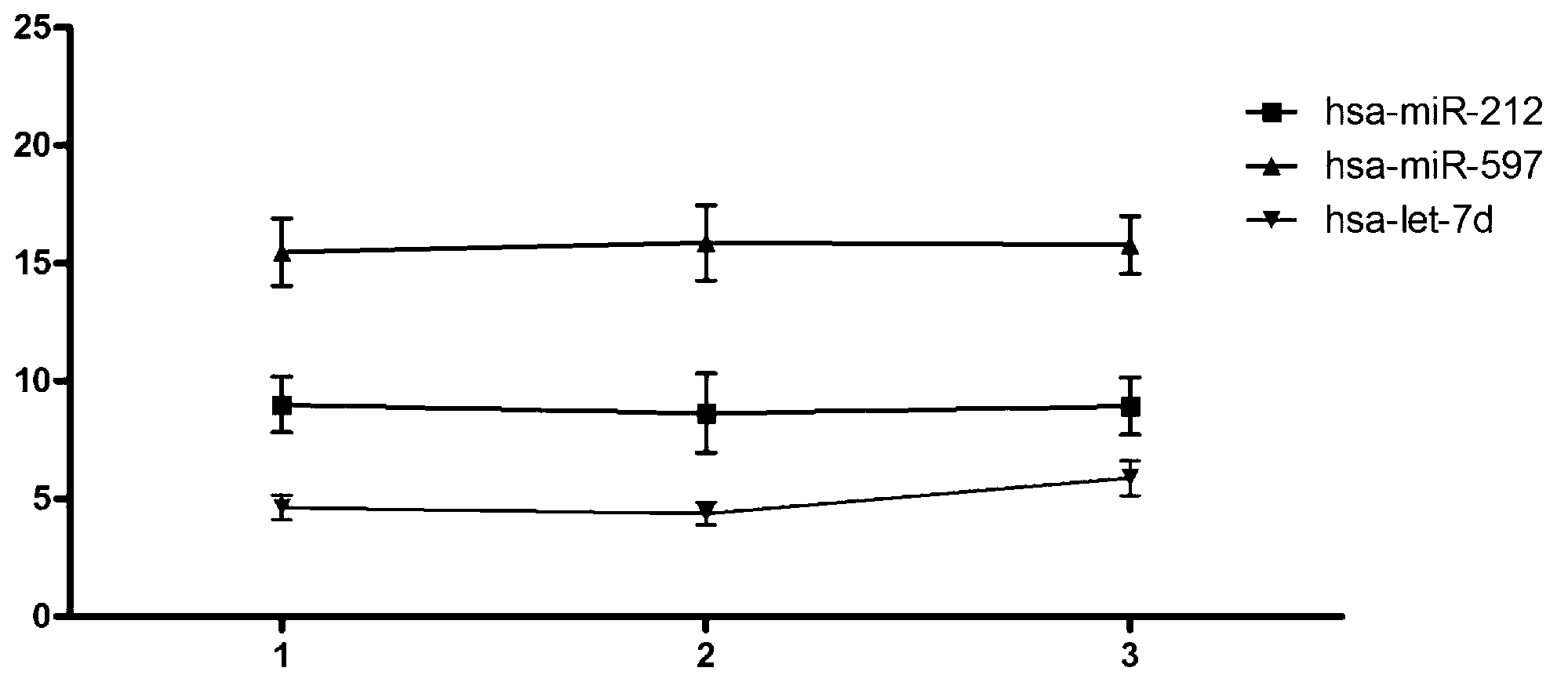
The invention belongs to the field of gene engineering and clinical medicine, and discloses a serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is more of hsa-miR-212, hsa-miR-597 and hsa-let-7d. The marker is specific and sensitive to fetal growth restriction, and can be used for preparing diagnostic or monitor reagents for fetal growth restriction.
Owner:夏彦恺
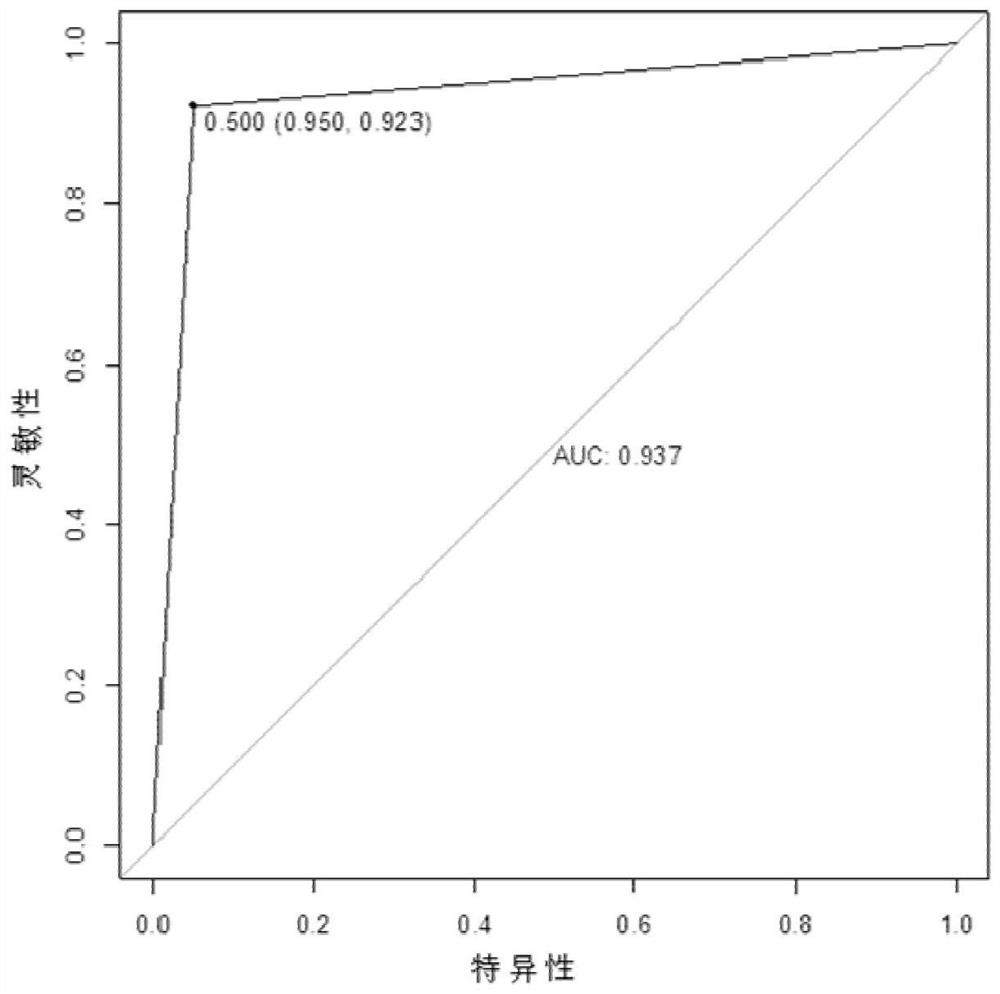
Esophageal squamous cell carcinoma early screening marker based on metabonomics and kit thereof
PendingCN113466370AGood distinguishing effectGood diagnosisComponent separationEsophageal squamous cell carcinomaMass screening
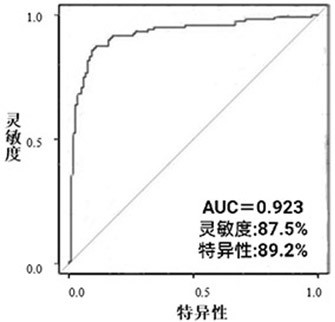
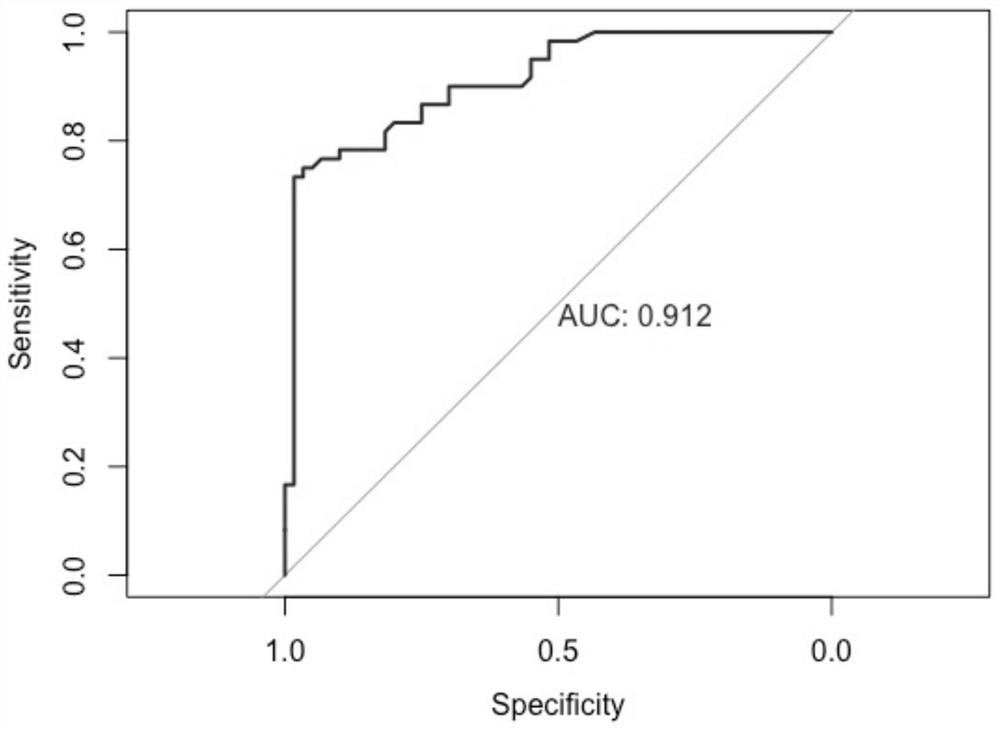
The invention belongs to the technical field of medical biology, and discloses an esophageal squamous cell carcinoma early screening marker based on metabonomics and a kit thereof. The marker for screening the esophageal squamous cell carcinoma, provided by the invention, is at least one of N-o-tolueneglycine, D-sorbitol and N-acetyl-D-mannosamine, and a detection reagent of the marker can be used for preparing a product for screening the esophageal squamous cell carcinoma. The invention also provides a kit for screening esophageal squamous carcinoma. The kit contains a detection reagent for detecting the marker. According to the kit, the esophageal squamous cell carcinoma, especially the early esophageal squamous cell carcinoma, can be effectively detected by detecting the expression levels of N-o-tolueneglycine, D-sorbitol and N-o-tolueneglycine in human serum; and when the three markers are combined, the detection sensitivity reaches up to 92.2%, the specificity reaches 98.0%, and the kit can be used for large-scale screening of asymptomatic crowds in high-incidence areas of esophageal squamous carcinoma and is beneficial to screening and early discovery of asymptomatic high-risk crowds.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Serum exosome miRNA marker related to liver cancer diagnosis, and application thereof
InactiveCN111206102AIncreased sensitivityImprove featuresOrganic active ingredientsMicrobiological testing/measurementAIDS diagnosisBiomarker (medicine)
The invention belongs to the field of biological diagnosis and medicine, and particularly relates to a serum exosome miRNA marker related to liver cancer diagnosis and application thereof. The markeris has-miRNA-21-5p which is used as a biomarker of liver cancer, whether a detected person has liver cancer or liver cancer risk or not is diagnosed by detecting the expression level of the marker, the marker has the characteristics of convenience in detection, small damage and the like, and meanwhile, the quantification is accurate, the sensitivity and the specificity of detection can be improved, and the has-miRNA-21-5p and a specific amplification primer thereof can be used for preparing a liver cancer diagnosis kit, so that the rapid and auxiliary diagnosis can be conveniently carried outat the early stage, and a basis is provided for further deeply examining or rapidly and accurately mastering the disease state of a patient by a clinician.
Owner:NANJING DRUM TOWER HOSPITAL
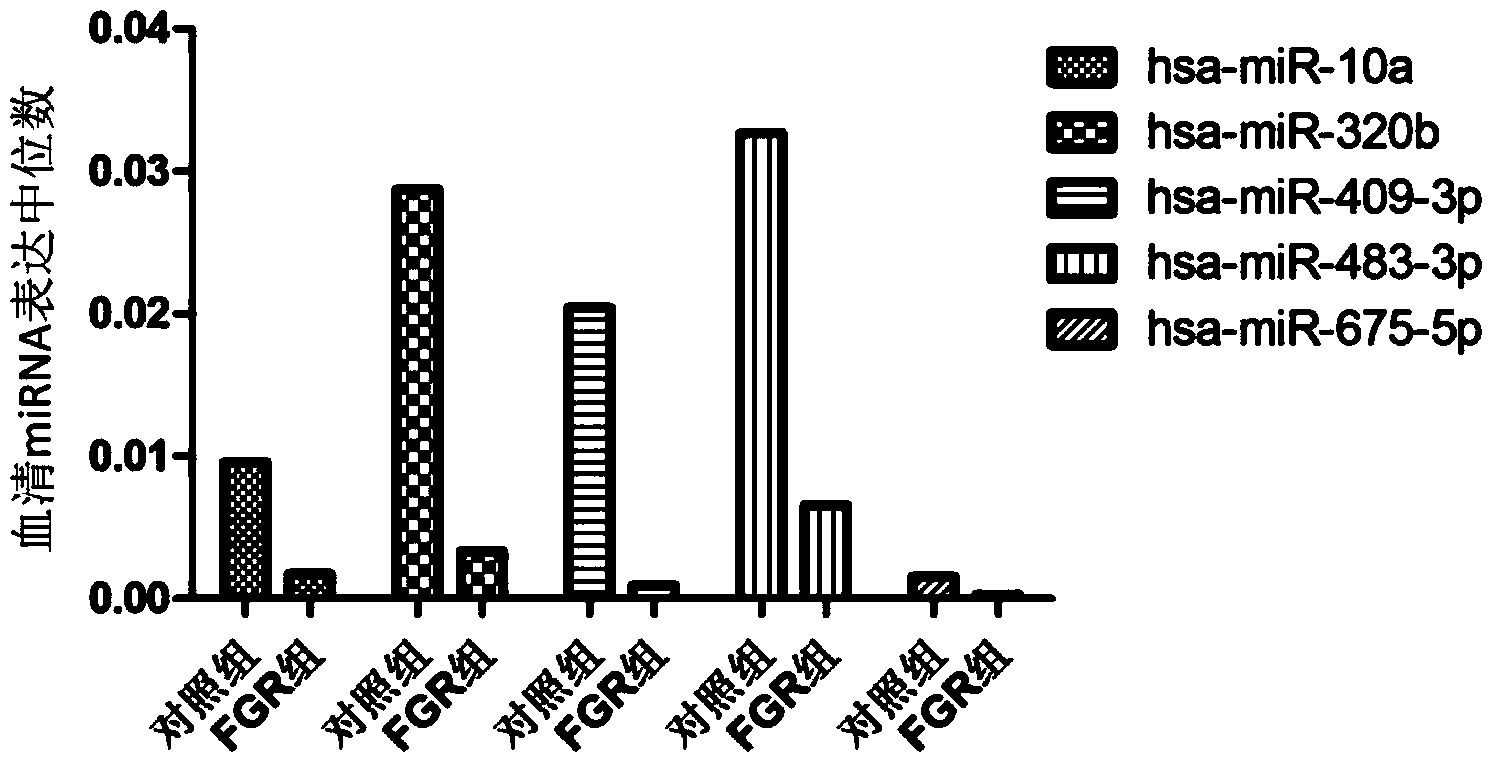
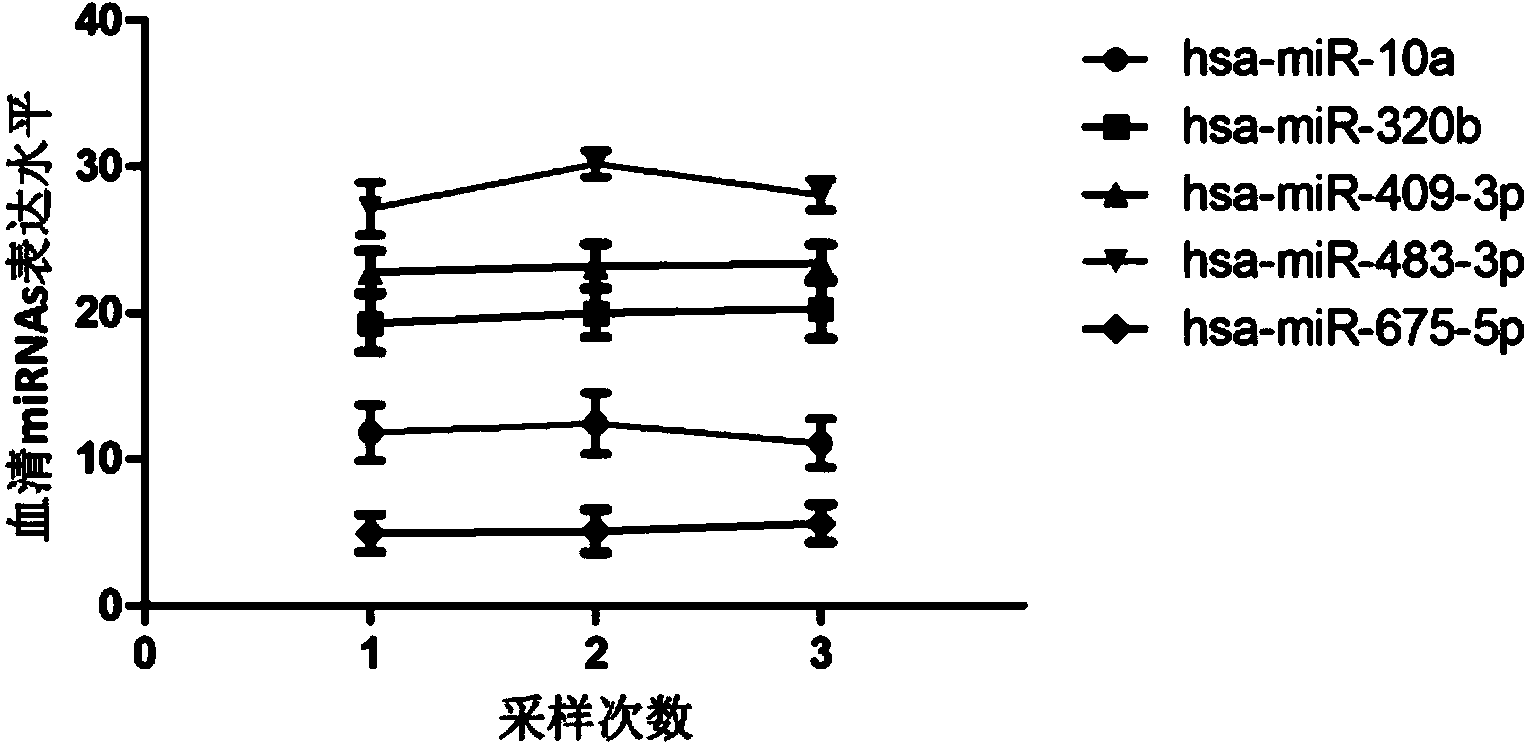
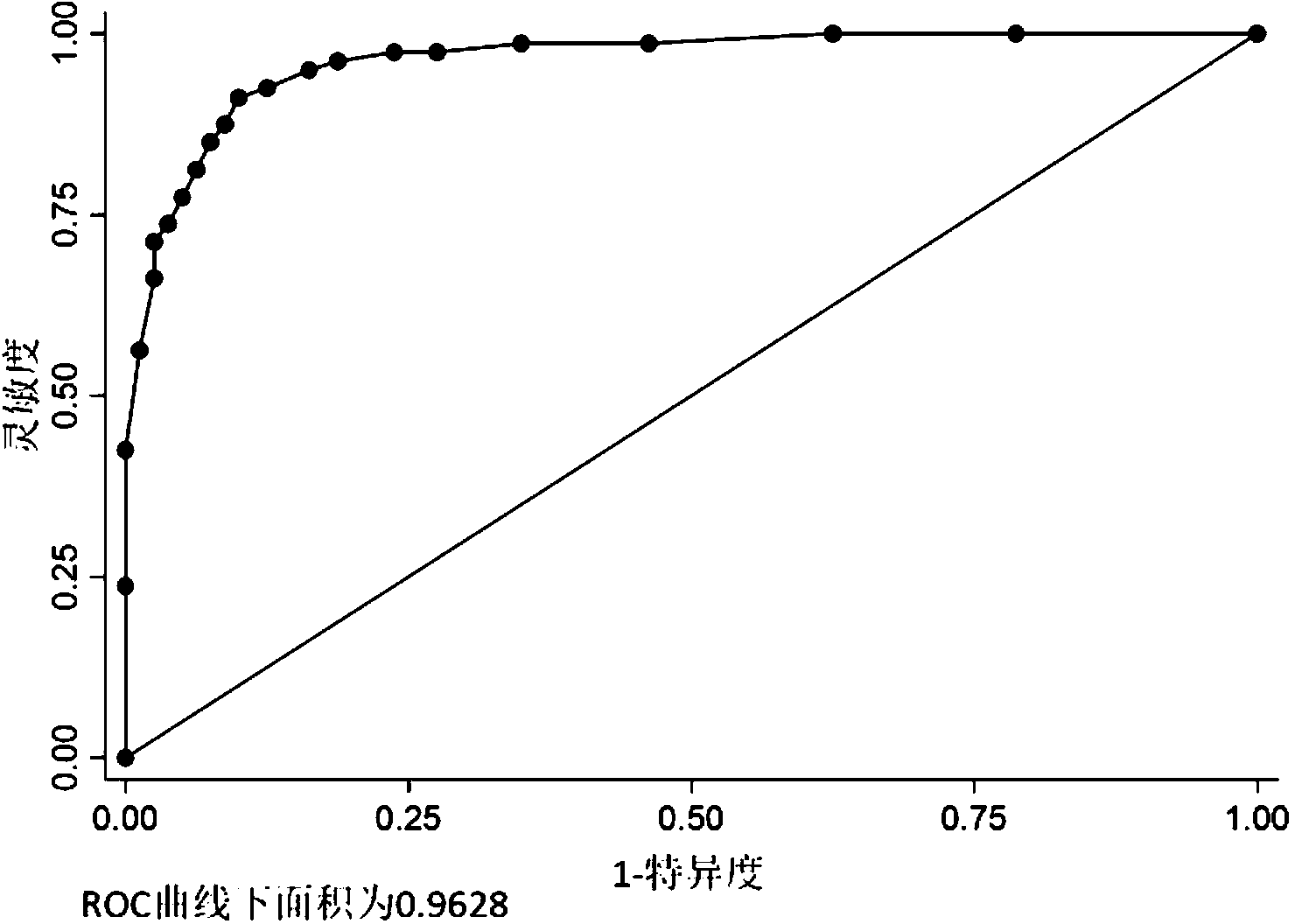
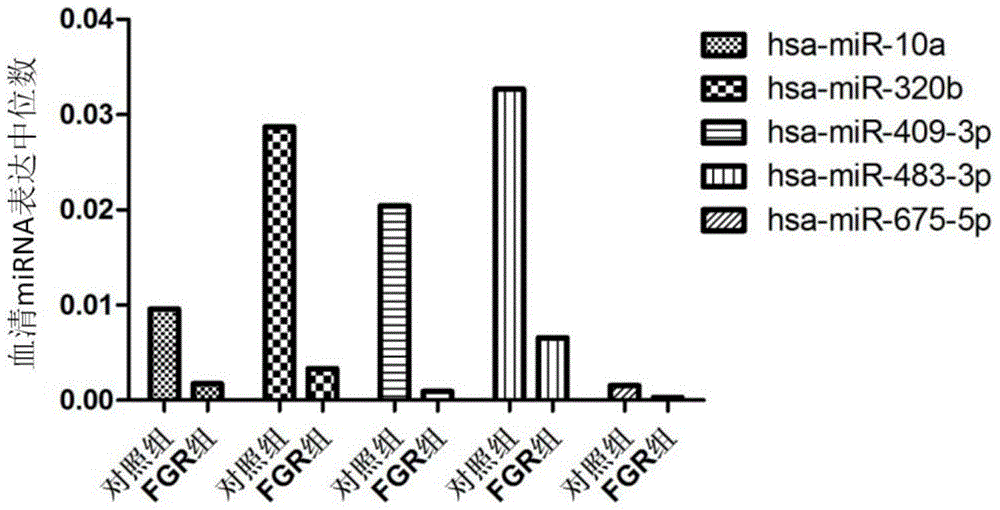
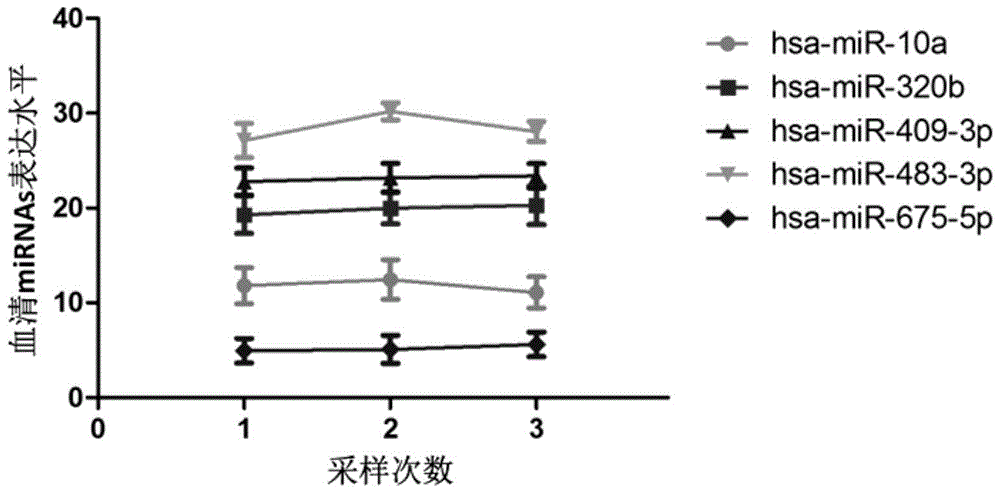
Tiny serum ribonucleic acid marker related to human fetal growth restriction and application thereof
ActiveCN103757017AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationFetal growthHuman fetal
The invention belongs to the fields of genetic engineering and reproductive medicine, and discloses a tiny serum ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is a plurality of hsa-miR-10a, hsa-miR-320b, hsa-miR-409-3p, hsa-miR-483-3p and hsa-miR-675-5p. The marker has specificity and sensibility on the fetal growth restriction, can be applied to preparation of the kit for diagnosing and monitoring the fetal growth restriction, can repeatedly detect, and is easy to dynamically monitor the fetal growth restriction degree.
Owner:NANJING MEDICAL UNIV
Application of serum exosomal miRNA markers in the early diagnosis of endemic arsenicosis
InactiveCN106435004BEasy extractionReduce the amount of serum drawnMicrobiological testing/measurementDNA/RNA fragmentationSerum igeExosomal mirnas
The invention belongs to the field of gene engineering and clinical medicine and particularly relates to an endemic arsenism early diagnosis associated serum exosome miRNAs marker and application thereof. The marker is a combination of has-miR-155 and has-miR-191. The marker and a primer thereof can be used for preparation of a kit for early diagnosis of endemic arsenism.
Owner:NANJING MEDICAL UNIV
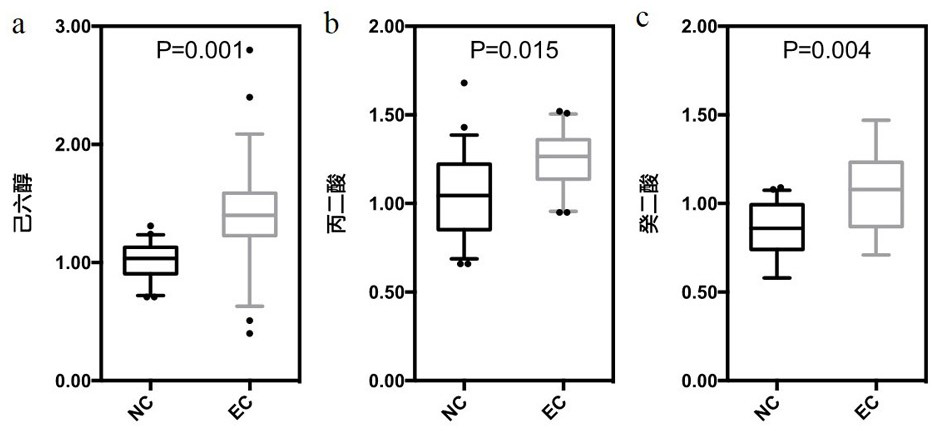
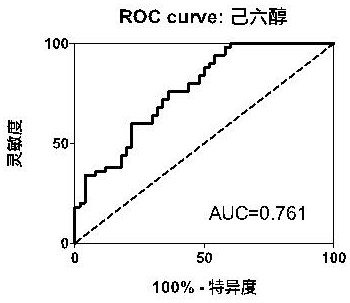
Marker and kit for diagnosing early esophageal cancer
PendingCN113777181ADiagnostic test worksEfficient detectionComponent separationMaterial analysis by electric/magnetic meansDiseaseMalonic acid
The invention belongs to the field of disease detection and diagnosis, and particularly discloses a marker and a kit for diagnosing early esophageal cancer. The marker for diagnosing the early esophageal cancer is at least one of hexanehexol, malonic acid and sebacic acid, and a detection reagent of the marker can be used for preparing an early esophageal cancer diagnosis product. The invention further provides the kit for diagnosing the early esophageal cancer, the kit contains the detection reagent for detecting the mentioned marker, and the detection reagent is a reagent for detecting the marker in a sample through chromatography, mass spectrometry or chromatography-mass spectrometry. The esophageal cancer can be effectively detected by detecting the expression levels of hexanehexol, malonic acid and sebacic acid in human serum, the detection sensitivity is as high as 84%, the specificity reaches 76%, and the kit and the marker can be used for large-scale screening of asymptomatic crowds in esophageal cancer high-incidence areas and are beneficial to screening and early discovery of asymptomatic high-risk crowds.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Related serum microribonucleic acid marker for human severe preeclampsia and application of marker
ActiveCN103205430BEasy to monitorHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPhysiologyGenetic engineering
The invention belongs to the fields of genetic engineering and clinical medicine, and discloses a related serum microribonucleic acid marker for human severe preeclampsia and application of the marker. The marker is selected from more of hsa-miR-193a-5p, hsa-miR-424 and hsa-miR-10a. The marker has the specificity and sensitivity on a sufferer with severe preeclampsia, and can be used for preparing a diagnosis or monitoring kit for severe preeclampsia.
Owner:夏彦恺
Serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof
ActiveCN103194542BHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationFetal growthHuman fetal
The invention belongs to the field of gene engineering and clinical medicine, and discloses a serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is more of hsa-miR-212, hsa-miR-597 and hsa-let-7d. The marker is specific and sensitive to fetal growth restriction, and can be used for preparing diagnostic or monitor reagents for fetal growth restriction.
Owner:夏彦恺
Plasma miRNA markers associated with common congenital intestinal aganglionosis and its application
ActiveCN103805603BEasy to monitorHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDynamic monitoringBlood plasma
The invention belongs to the fields of genetic engineering and clinical medicines, and discloses a plasma miRNA (micro ribonucleic acid) marker associated with common type congenital intestinal canal total colonic aganglionosis and an application thereof. The marker is selected from more of hsa-miR-31, hsa-miR-147 and hsa-miR-206. The marker has specificity and sensitivity to the common type congenital intestinal canal total colonic aganglionosis, can be used for the preparation of reagents for diagnosing or monitoring the common type congenital intestinal canal total colonic aganglionosis, can avoid invasive diagnosis, can be used for screening and diagnosis in an early stage, and can be used for detecting repeatedly and is easy for dynamic monitoring.
Owner:NANJING CHILDRENS HOSPITAL AFFILIATED TO NANJING MEDICAL UNIV
Plasma metabolic small molecule markers related to human non-small cell lung cancer and their application
InactiveCN105203683BRepair inhibitionIncrease contractilityComponent separationMetaboliteConfidence interval
The invention belongs to the field of analytical chemistry and clinical medicine and relates to a plasma metabolization micromolecule marker related to the human non-small-cell lung cancer and an application of the plasma metabolization micromolecule marker. The plasma metabolization micromolecule marker related to the human non-small-cell lung cancer is one or more of cortisol, corticosterone and 4-methoxyphenylacetic acid. The plasma metabolization micromolecule marker is prepared from cortisol, corticosterone and 4-methoxyphenylacetic acid. The content range (95% confidence interval) of cortisol is 0.00018-0.00067, the content range (95% confidence interval) of corticosterone is 0.000029-0.00010, the content range (95% confidence interval) of 4-methoxyphenylacetic acid is 0.000015-0.000022, and metabolite can prompt occurrence of tumors within the ranges. The horizontal range, corresponding to a normal group, of cortisol is 0.0030-0.0037, the horizontal range, corresponding to the normal group, of corticosterone is 0.00044-0.00056, and the horizontal range, corresponding to the normal group, of 4-methoxyphenylacetic acid is 7.39 E-07-2.09 E-06. The plasma metabolization micromolecule marker is a novel biomarker, compared with a traditional protein biomarker, the relevance between the marker and the disease outcome is higher, and the plasma metabolization micromolecule marker is stable, minimally invasive, easy to detect and accurate in quantitation.
Owner:JIANGSU PROVINCE HOSPITAL
Seminal plasma micro ribonucleic acid marker related to spermatogenesis deficiency and application thereof
InactiveCN101633925BIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationPhysiologyMicroRNA
The invention belongs to the field of gene engineering and reproductive medicines, and discloses a seminal plasma micro ribonucleic acid marker related to spermatogenesis deficiency and application thereof. The marker is selected from one or more of SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4, has specificity and sensitivity to the spermatogenesis deficiency, can be used for preparing a reagent for diagnosing or monitoring the spermatogenesis deficiency, can avoid invasive diagnosis, can carry out repeated detection, and is easy to dynamically monitor the degree of the spermatogenesis deficiency.
Owner:NANJING MEDICAL UNIV
Marker and kit for cardia cancer diagnosis
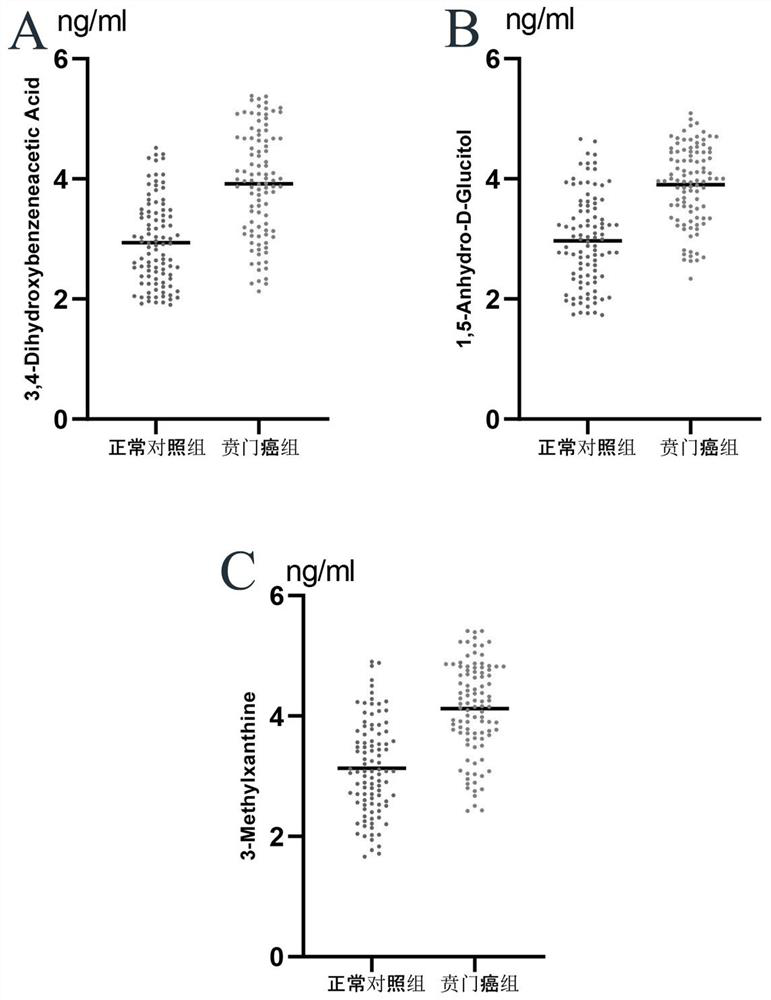
PendingCN113433239AGood distinctionEasy diagnosisComponent separationMaterial analysis by electric/magnetic meansCancers diagnosisGastric Cardia Carcinoma
The invention belongs to the technical field of medical biology and particularly discloses a marker and a kit for cardia cancer diagnosis. The marker for cardia cancer diagnosis, provided by the invention, is at least one of 3, 4-Dihydroxybenzeneacetic Acid, 1, 5-Androro-D-Glucitol and 3-Methylxanthine, and a detection reagent of the marker can be used for preparing a cardia cancer diagnosis product. The invention also provides a kit for cardia cancer diagnosis, the kit contains a detection reagent for detecting the marker in the first aspect, and the detection reagent is a reagent for detecting the marker in a sample through chromatography-mass spectrometry or chromatography-mass spectrometry. The cardia cancer can be effectively detected by detecting expression levels of 3, 4-Dihydroxybenenoic Acid, 1, 5-Androdro-D-Glucitol and 3-Methylxanthine in human serum, detection sensitivity is as high as 90.0%, specificity reaches 91.0%, and the kit can be used for large-scale screening of asymptomatic crowds in a cardia cancer high-incidence area and is beneficial to screening and early discovery of asymptomatic high-risk crowds.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV +1
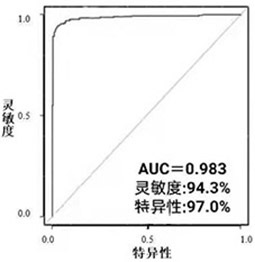
Marker and detection kit for cardia cancer screening
ActiveCN113447586AEfficient detectionGood distinctionComponent separationGastric Cardia CarcinomaMass Spectrometry-Mass Spectrometry
The invention belongs to the technical field of medical biology, and particularly discloses a marker and a detection kit for cardia cancer screening. The marker for screening the cardia cancer, which is provided by the invention, is at least one of Xanthurenic Acid and 2-Hydroxy-2-methylbutyric acid, and a detection reagent of the marker can be used for preparing a product for screening the cardia cancer. The invention also provides the kit for screening cardia cancer, the kit contains a detection reagent for detecting the marker, and the detection reagent is a reagent for detecting the marker in a sample through chromatography-mass spectrometry or chromatography-mass spectrometry. The cardia cancer can be effectively detected by detecting the expression levels of Xanthurenic Acid and 2-Hydroxy-2-methylbutyric acid in human serum, the detection sensitivity reaches up to 94.3%, the specificity reaches 97.0%, and the kit can be used for large-scale screening of asymptomatic crowds in a cardia cancer high-incidence area and is beneficial to screening and early discovery of asymptomatic high-risk crowds.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV +1
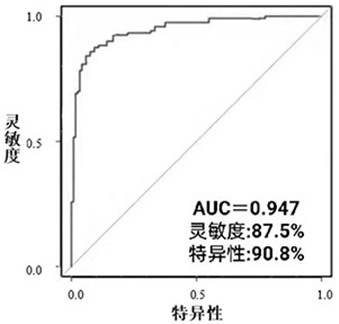
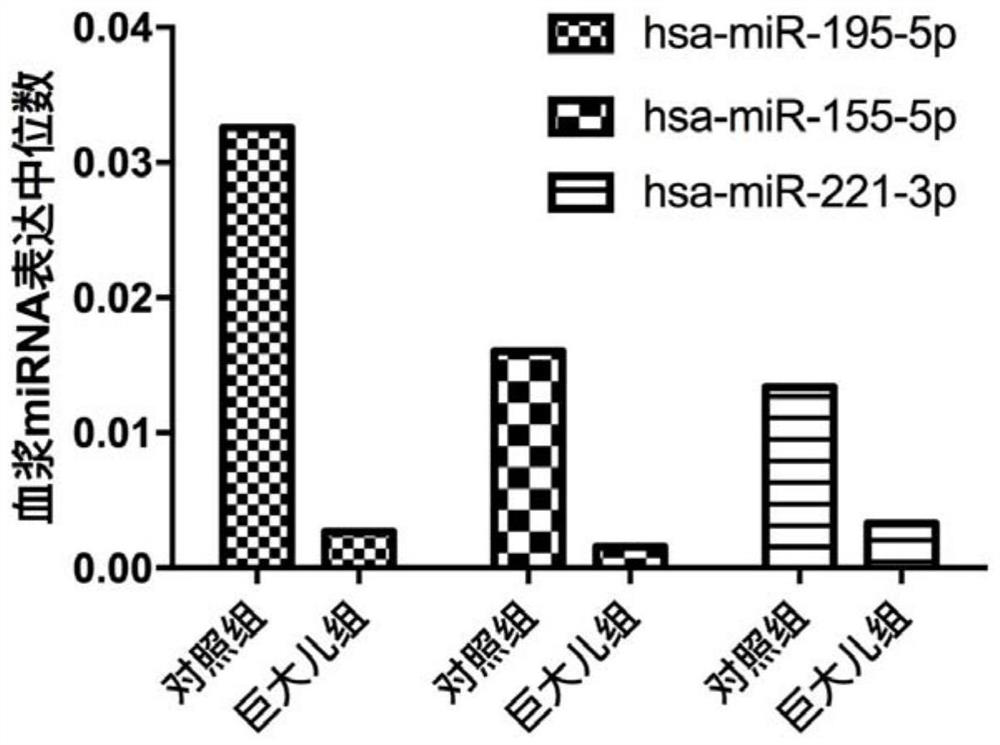
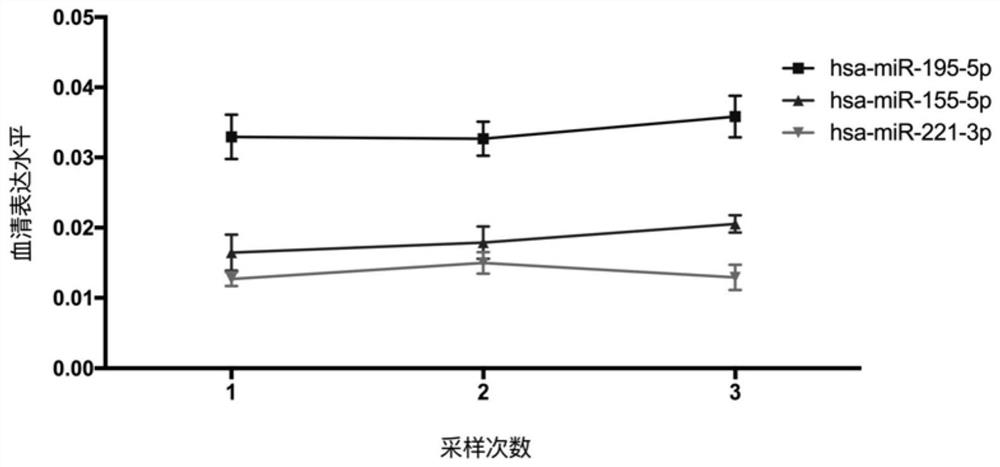
Detecting and/or predicting miRNA markers or combinations thereof in human macrosomia and applications thereof
ActiveCN108546752BHigh sensitivityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiagnosis earlyGenetic engineering
The invention discloses a miRNA marker related to human macrosomia or its combination and application, belonging to the fields of genetic engineering and clinical medicine. The marker is selected from one or more of hsa-miR-195-5p, hsa-miR-155-5p, and hsa-miR-221-3p. The marker has specificity and sensitivity for macrosomia, and can be used to prepare a kit for the early diagnosis or monitoring of macrosomia, which can be screened and diagnosed at an early stage, and can be repeatedly detected and easily monitored dynamically.
Owner:NANJING HANWEI PUBLIC HEALTH RES INST CO LTD
Plasma micro-ribonucleic acid (miRNA) marker related with human Hirschsprung's disease and application of miRNA marker
ActiveCN102358900BEasy diagnosisHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMedicine
The invention belongs to the fields of gene engineering and clinical medicine, and discloses a plasma micro-ribonucleic acid (miRNA) marker related with a human Hirschsprung's disease and application of the miRNA marker. The marker is selected from multiple kinds of hsa-miR-34b, hsa-miR-31*, hsa-miR-141 and hsa-miR-194. The marker has specificity and sensitivity on the Hirschsprung's disease, canbe used for preparing a reagent for diagnosing or monitoring the Hirschsprung's disease, can avoid invasive diagnosis, can be used for screening and diagnosis at the early stage, can be repeatedly detected, and is easy in dynamic monitoring.
Owner:NANJING MEDICAL UNIV
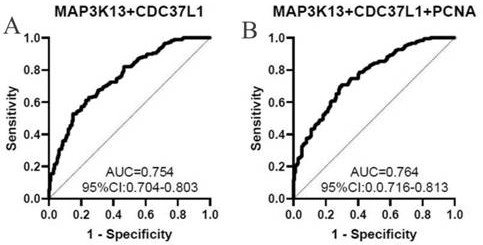
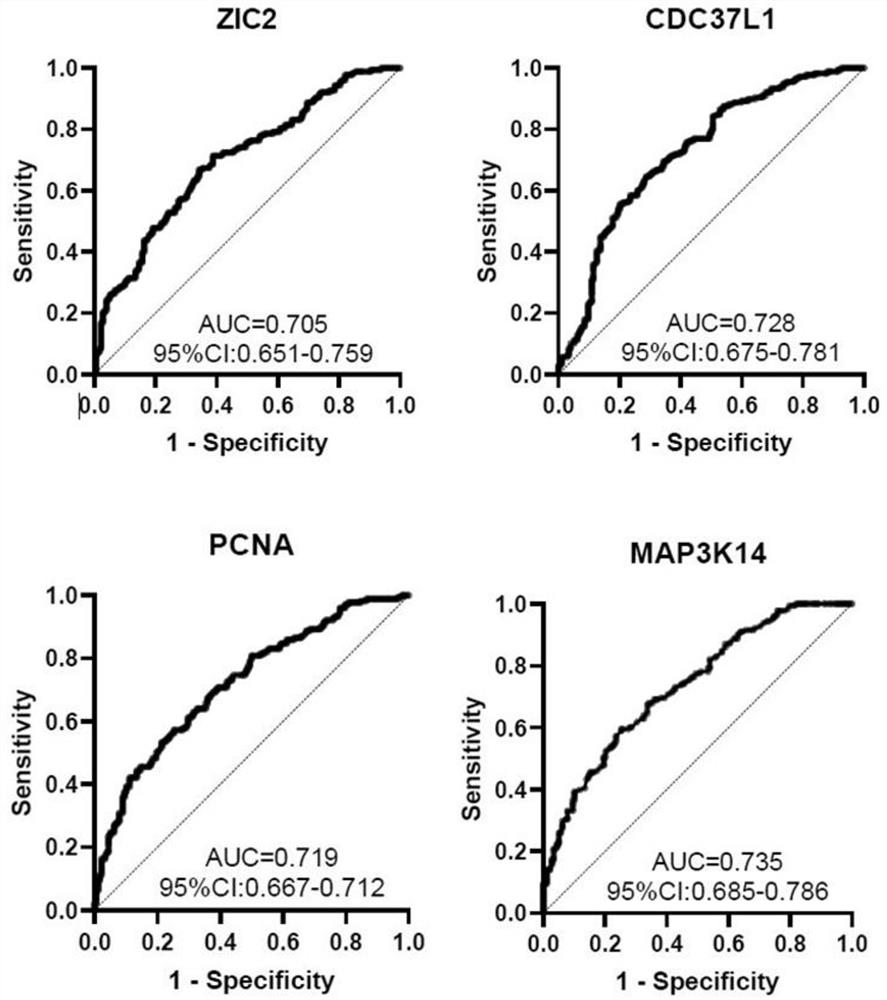
Biomarker for liver cancer diagnosis and detection kit
ActiveCN114113611AGood distinctionHigh detection sensitivityBiological testingImmunoassaysAntigenAutoantibody
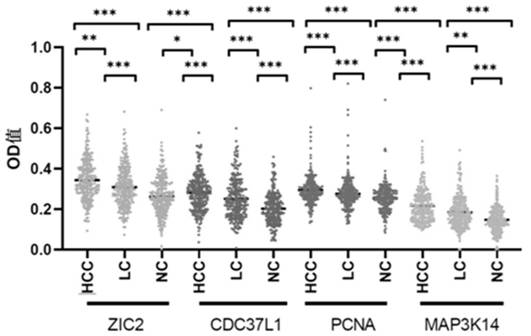
The invention belongs to the technical field of medical biology, and particularly discloses a biomarker for ovarian cancer diagnosis and a detection kit. The biomarker for liver cancer diagnosis provided by the invention is at least one of autoantibodies of anti-tumor related antigens ZIC2, CDC37L1, PCNA and MAP3K14, the expression level of the marker in serum of a liver cancer patient is higher than that of a normal person, and the difference has statistical significance. The invention also provides a kit for liver cancer diagnosis, the kit contains a reagent for detecting the biomarker, and the reagent is used for detecting the biomarker in a sample through enzyme-linked immunosorbent assay, a protein chip, immunoblotting or microfluidic immunoassay. By detecting the expression level of the biomarker in human serum, liver cancer patients and healthy people can be effectively distinguished, and the biomarker can be used for auxiliary diagnosis of liver cancer.
Owner:ZHENGZHOU UNIV
A serum microRNA marker related to the occurrence of human fetal growth restriction and its application
ActiveCN103757017BHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationSerum markersSerum ige
The invention belongs to the fields of genetic engineering and reproductive medicine, and discloses a tiny serum ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is a plurality of hsa-miR-10a, hsa-miR-320b, hsa-miR-409-3p, hsa-miR-483-3p and hsa-miR-675-5p. The marker has specificity and sensibility on the fetal growth restriction, can be applied to preparation of the kit for diagnosing and monitoring the fetal growth restriction, can repeatedly detect, and is easy to dynamically monitor the fetal growth restriction degree.
Owner:NANJING MEDICAL UNIV
Biomarker for diagnosing esophageal squamous carcinoma and detection kit
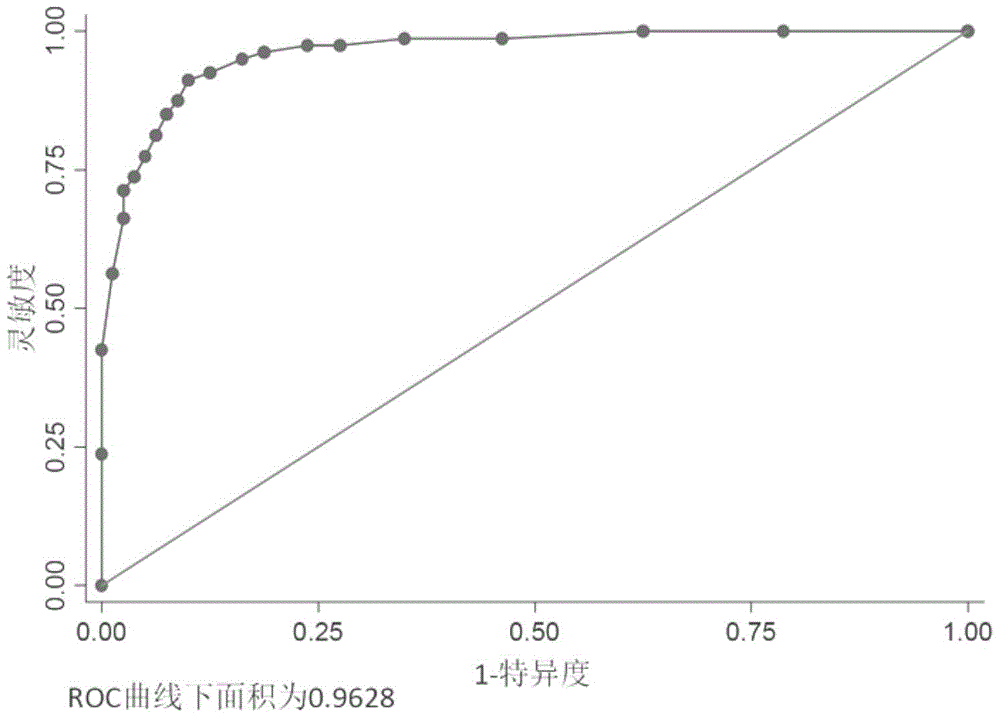
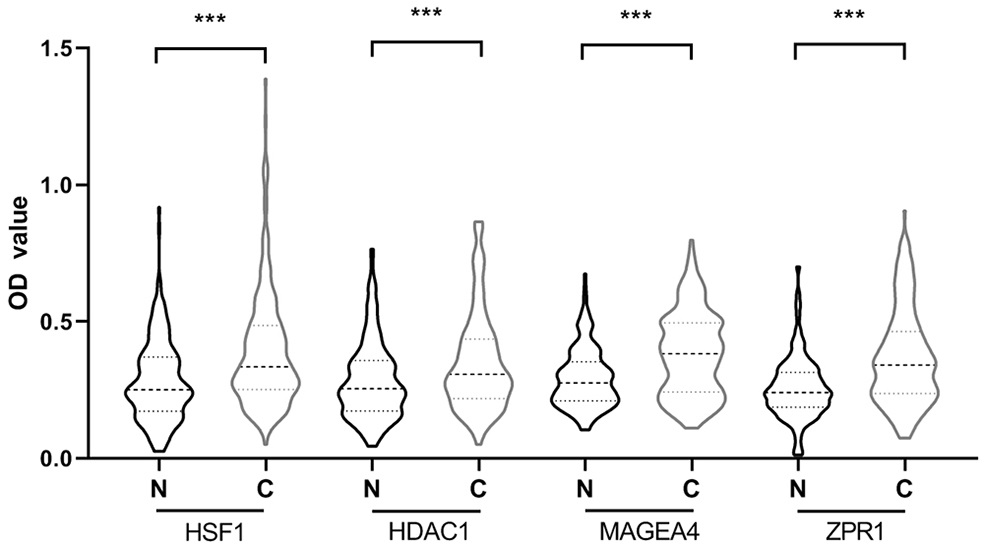
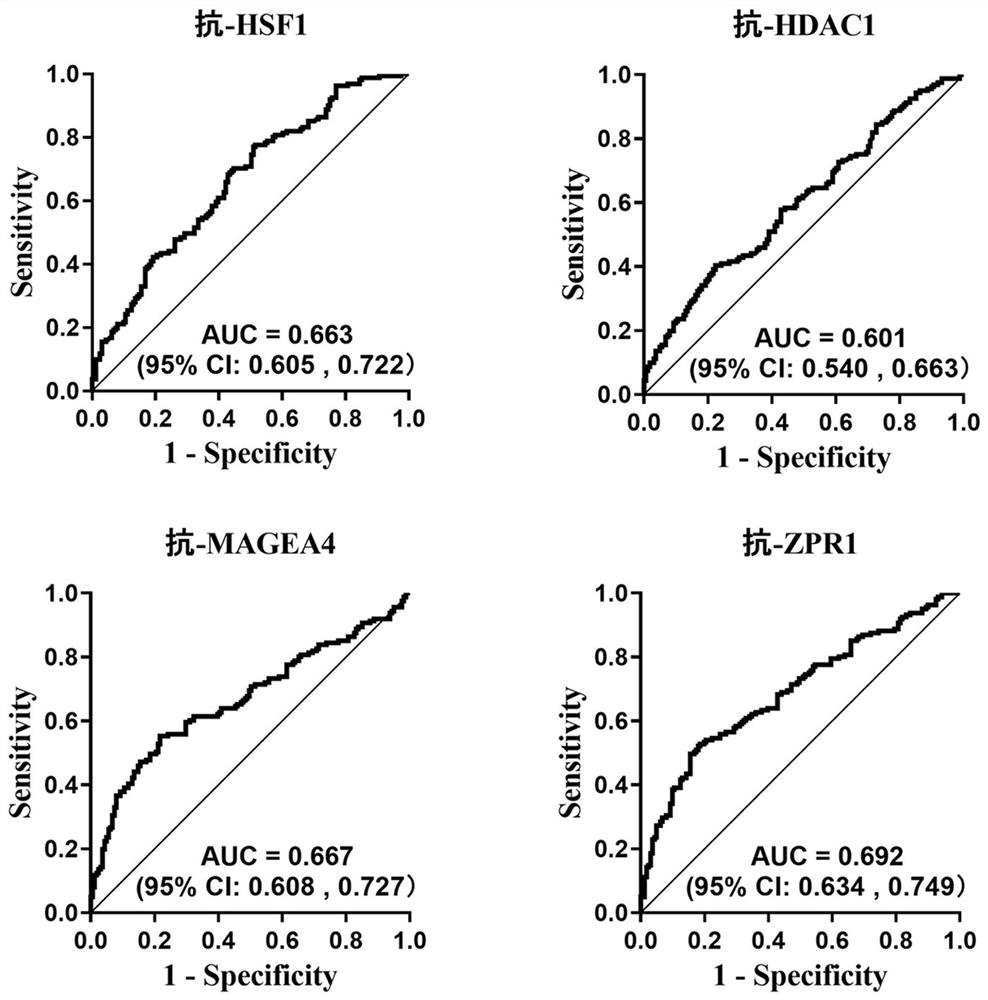
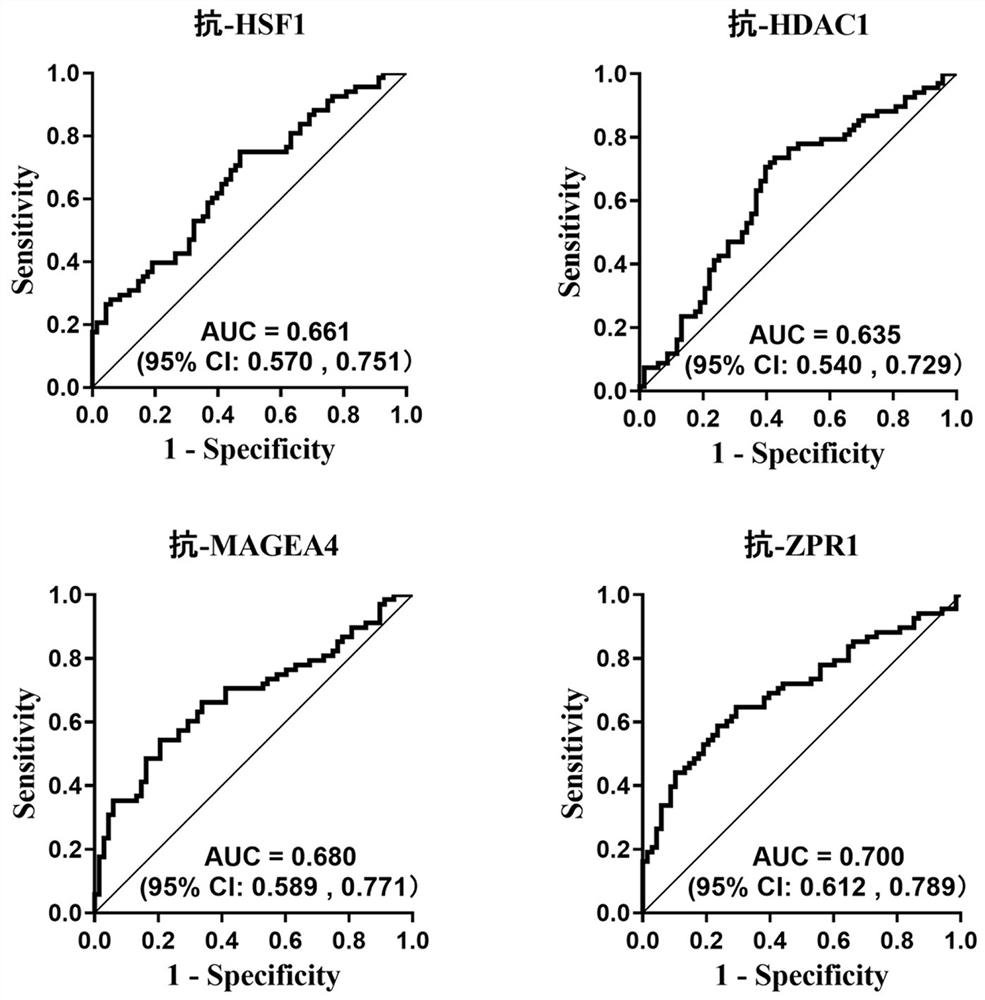
The invention belongs to the technical field of medical biology, and particularly discloses a biomarker for auxiliary diagnosis of esophageal squamous carcinoma and a detection kit. The biomarker for auxiliary diagnosis of esophageal squamous cell carcinoma provided by the invention is at least one of autoantibodies of anti-tumor related antigens ZPR1, HSF1, MAGEA4 and HDAC1, the expression level of the marker in serum of a patient with esophageal squamous cell carcinoma is higher than that of a normal person, and the difference has statistical significance. The invention also provides a kit for auxiliary diagnosis of esophageal squamous carcinoma, the kit contains a reagent for detecting the marker, and the reagent is used for detecting the biomarker in a sample through enzyme-linked immunosorbent assay, a protein chip, immunoblotting or microfluidic immunoassay. By detecting the expression level of the biomarker in human serum, esophageal squamous cell carcinoma patients and normal people can be effectively distinguished, and the marker can be used for esophageal squamous cell carcinoma diagnosis.
Owner:ZHENGZHOU UNIV
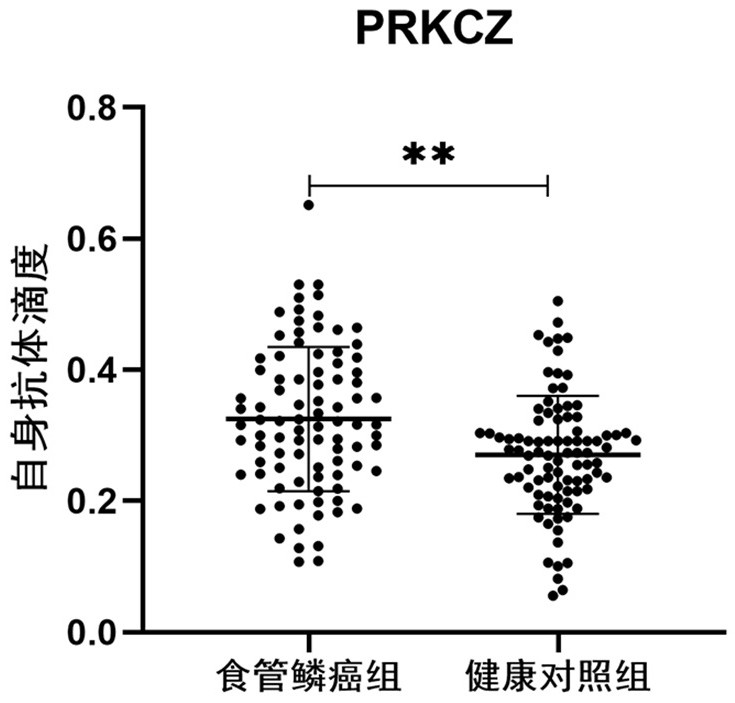
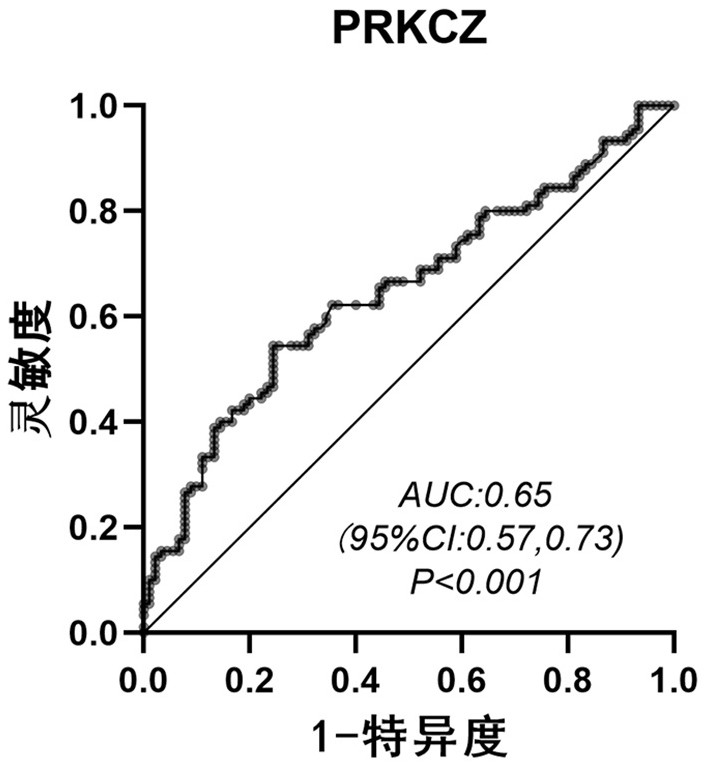
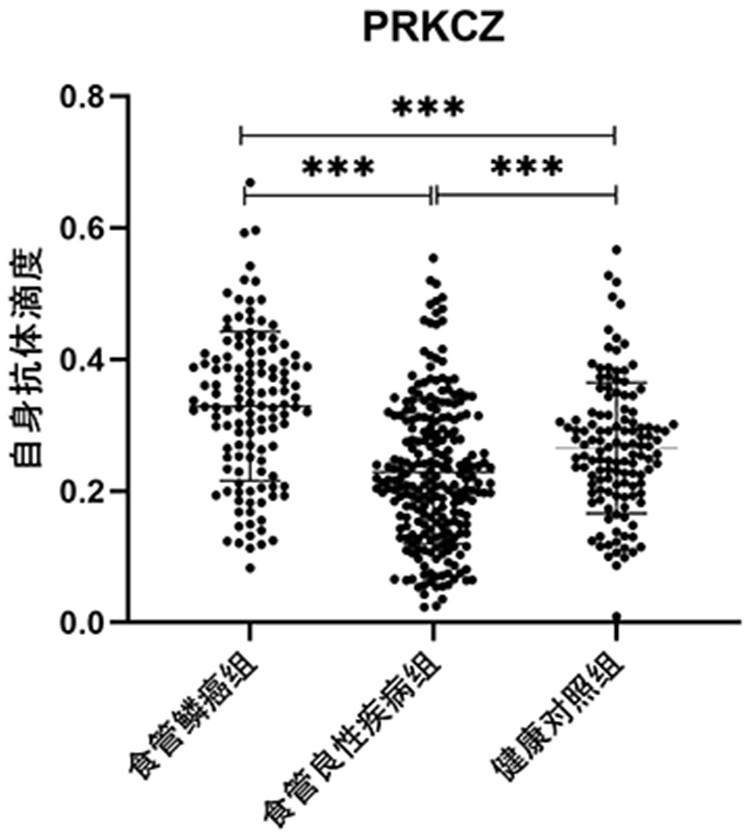
Application of PRKCZ autoantibody in auxiliary diagnosis of esophageal squamous carcinoma
ActiveCN113721021AAuxiliary diagnosis realizedReduce mortalityMaterial analysisAutoantibodyImmuno detection
The invention belongs to the technical field of medical biology, and particularly discloses a biomarker for auxiliary diagnosis of esophageal squamous carcinoma and a detection kit. The biomarker for auxiliary diagnosis of esophageal squamous cell carcinoma provided by the invention is an autoantibody of an anti-tumor associated antigen PRKCZ, the expression level of the marker in serum of a patient with esophageal squamous cell carcinoma is higher than that of a normal person, and the difference has statistical significance. The invention also provides a kit for auxiliary diagnosis of esophageal squamous carcinoma, the kit contains a reagent for detecting the marker, and the reagent is used for detecting the biomarker in a sample through enzyme-linked immunosorbent assay, a protein chip, immunoblotting or microfluidic immunoassay. By detecting the expression level of the anti-tumor associated antigen PRKCZ in human serum, patients with esophageal squamous carcinoma and normal people can be effectively distinguished, and the kit can be used for auxiliary diagnosis of esophageal squamous carcinoma.
Owner:ZHENGZHOU UNIV
Serum/plasma miRNA markers associated with type 2 diabetic retinopathy and their application
ActiveCN107385035BIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiabetes retinopathyDiabetic retina
The present invention provides a serum / plasma miRNA marker related to type 2 diabetic retinopathy and use thereof. The serum / plasma miRNA marker related to the type 2 diabetic retinopathy is one or a combination of two of hsa-let-7a-5p, hsa-miR-novel-chr5_15976, hsa-miR-28-3p, has-miR-20a-5p, has-miR-151a-5p, has-miR-148a-3p and has-miR-223s-3p. The serum / plasma miRNA marker related to the type 2 diabetic retinopathy is stable and can be used to early assist to diagnose whether a diabetic patient is combined with DR, and the disease progression of the diabetic retinopathy patient can be dynamically monitored, and the serum / plasma miRNA marker can be used to prepare an early diagnosis product of type 2 diabetes and the diabetic retinopathy.
Owner:SHENZHEN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com