Serum/plasma miRNA marker related to type 2 diabetic retinopathy and use thereof
A technology for type 2 diabetes and retinopathy, applied in the fields of genetic engineering and clinical medicine, can solve the problem of inability to diagnose type 2 diabetic retinopathy at an early stage, and achieve the effects of early diagnosis, improved sensitivity and specificity, and accurate quantitative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
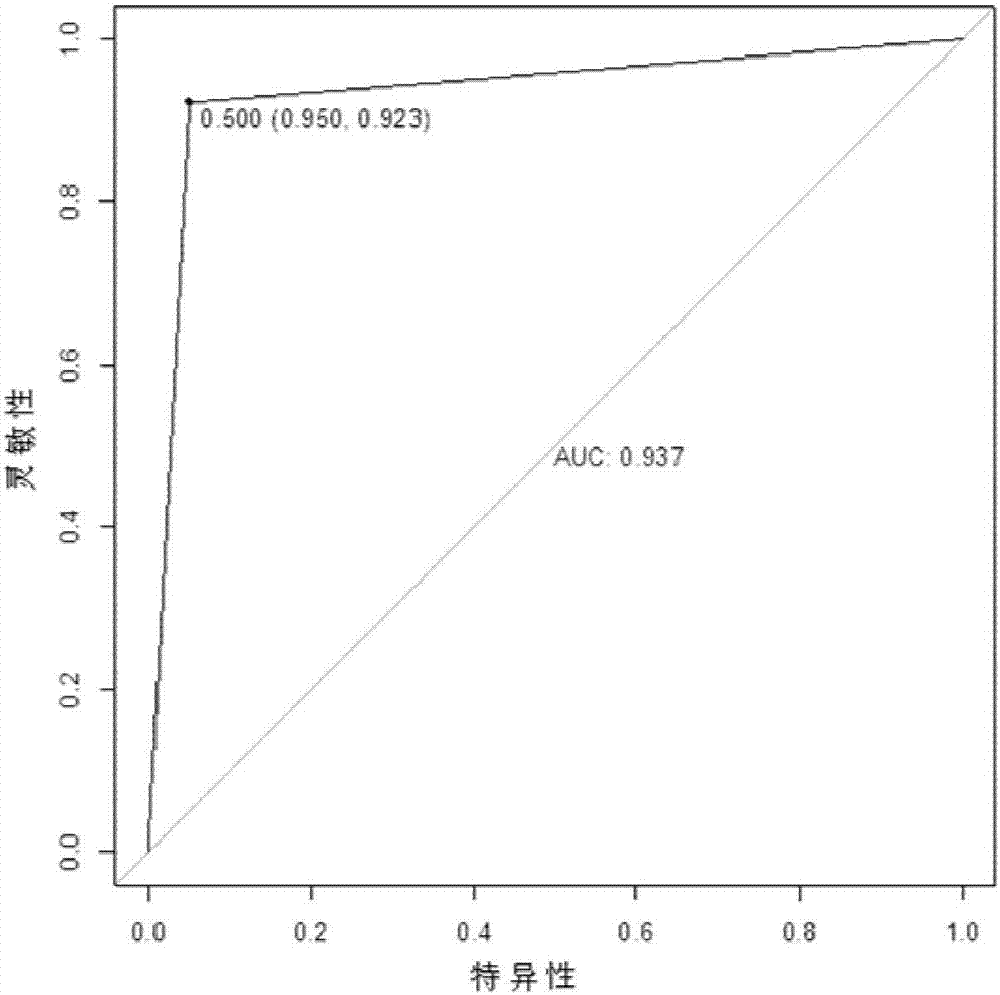
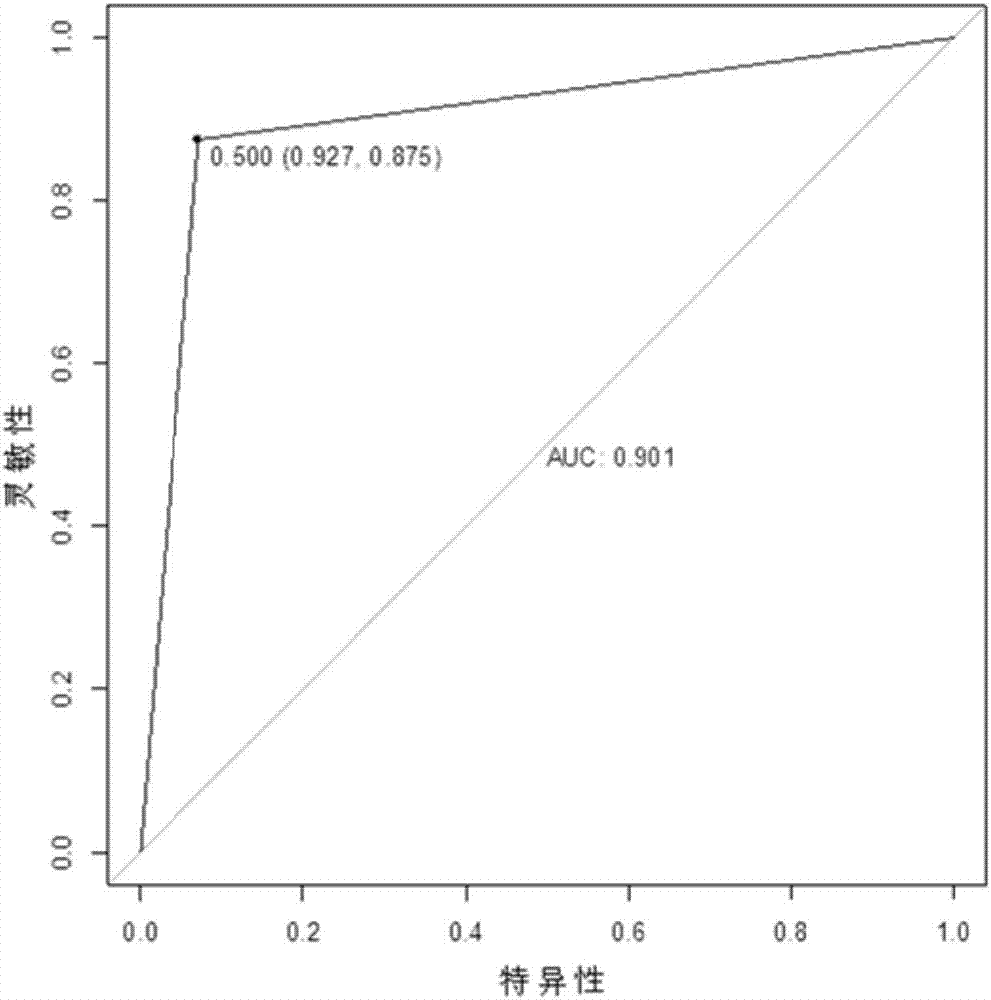
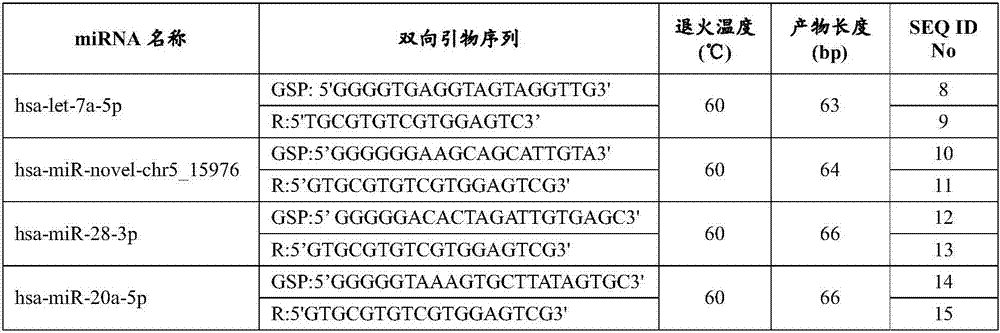
[0026] In one aspect, the embodiments of the present invention provide a serum / plasma miRNA marker associated with type 2 diabetic retinopathy. The serum / plasma miRNA markers associated with type 2 diabetic retinopathy are hsa-let-7a-5p, hsa-miR-novel-chr5_15976, hsa-miR-28-3p, has-miR-20a-5p, has - One or a combination of two or more of miR-151a-5p, has-miR-148a-3p and has-miR-223-3p. It has been verified that these miRNA markers can effectively diagnose type 2 diabetic retinopathy, especially the early stage (I-II stage) type 2 diabetic retinopathy.
[0027] After further verification, as an ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com