Marker and kit for cardia cancer diagnosis
A technology of markers and kits, applied in the field of medicine and biology, can solve the problems of lack of specificity in detection, and achieve good diagnostic and distinguishing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Screening of Serum Differential Metabolic Markers of Cardiac Cancer
[0035] 1. Experimental samples
[0036] According to strict screening and exclusion criteria, 100 age- and gender-matched healthy subjects (normal control group) and 100 cardiac cancer patients (cardia cancer group) were collected from the First Affiliated Hospital of Zhengzhou University.
[0037] The inclusion criteria for healthy subjects are: no cardiovascular, respiratory, liver, kidney, gastrointestinal, endocrine, blood, mental, or nervous system diseases and history of the above diseases, no acute or chronic diseases, no evidence of any tumor-related , no history of drug allergy, and the results of clinical laboratory tests were within the normal reference range at the time of screening.
[0038] The inclusion criteria for patients with cardiac cancer were as follows: patients with cardiac cancer confirmed by endoscopy and histopathology had not received radiotherapy or chemotherap...
Embodiment 2
[0065] Example 2: Evaluation of the ability of differential metabolite diagnosis to distinguish cardiac cancer patients from healthy individuals
[0066] (1) The ability of a single differential metabolite diagnosis to distinguish cardiac cancer patients from normal individuals:
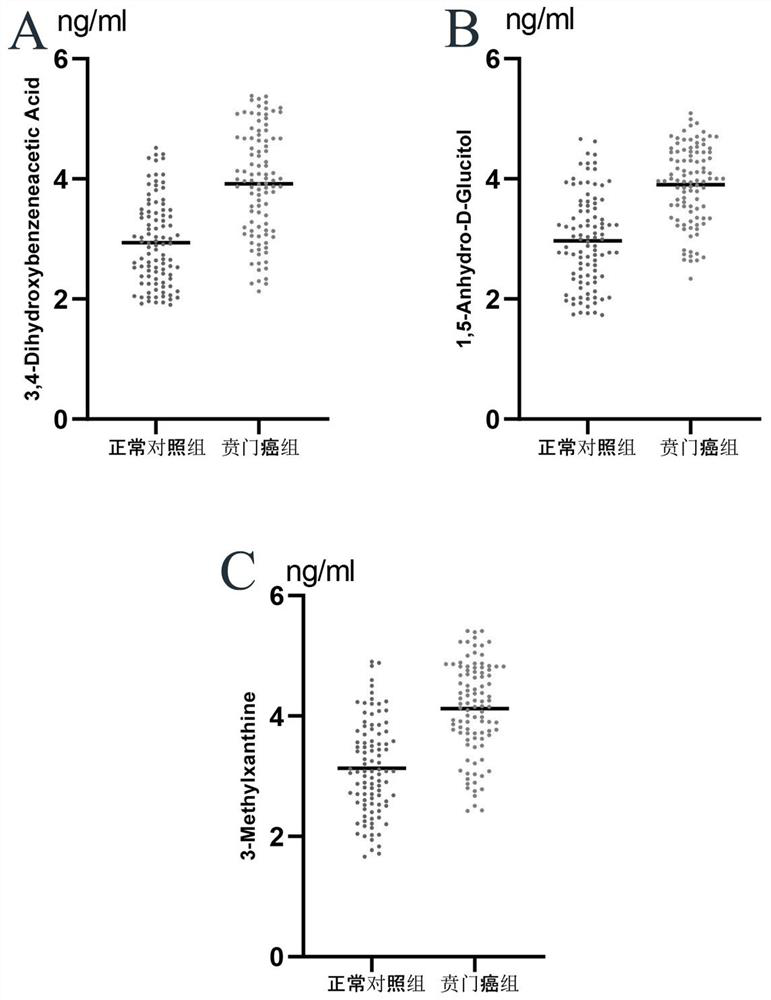
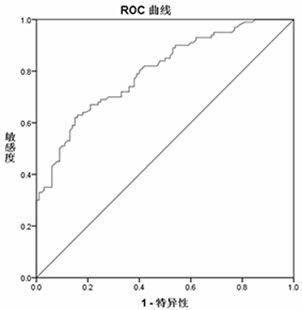
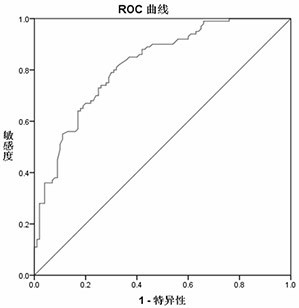
[0067] 3,4-Dihydroxybenzoneacetic Acid, 1,5- The content data of Anhydro-D-Glucitol and 3-Methylxanthine were analyzed, and the receiver operating curve (ROC curve) was used to evaluate the ability of each differential metabolite to diagnose and distinguish cardiac cancer patients from normal people. 3,4-Dihydroxybenzoneacetic Acid, 1,5-Anhydro-D-Glucitol, and 3-Methylxanthine alone can diagnose the ROC curves for differentiating cardiac cancer patients from normal subjects. figure 2 , image 3 , Figure 4 shown. According to the ROC curve, the area under the curve (AUC), sensitivity and specificity of the ROC curve of each differential metabolite was counted, and the results are shown in Table ...
Embodiment 3
[0087] Example 3: Application of Three Differential Metabolites in Cardiac Cancer Screening
[0088] 1. Collection of serum samples
[0089] According to strict screening and exclusion criteria, 500 healthy subjects (normal control group) and 500 cardiac cancer patients (cardia cancer group) were collected from the First Affiliated Hospital of Zhengzhou University after age and sex matching.
[0090] The inclusion criteria for healthy subjects are: no cardiovascular, respiratory, liver, kidney, gastrointestinal, endocrine, blood, mental, or nervous system diseases and history of the above diseases, no acute or chronic diseases, no evidence of any tumor-related , no history of drug allergy, and the results of clinical laboratory tests were within the normal reference range at the time of screening.
[0091] The inclusion criteria for patients with cardiac cancer were as follows: patients with cardiac cancer confirmed by endoscopy and histopathology had not received radiotherap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com