Method for removing residual dna in Japanese encephalitis vaccine products by anion exchange chromatography
An exchange chromatography, anion technology, applied in the direction of resistance to vector-borne diseases, virus antigen components, etc., can solve the problems of large side effects of vaccines, low removal rate of impurity proteins, and high host DNA content, to achieve removal content and improve product quality. and pass rate, the effect of breaking through the quality bottleneck
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
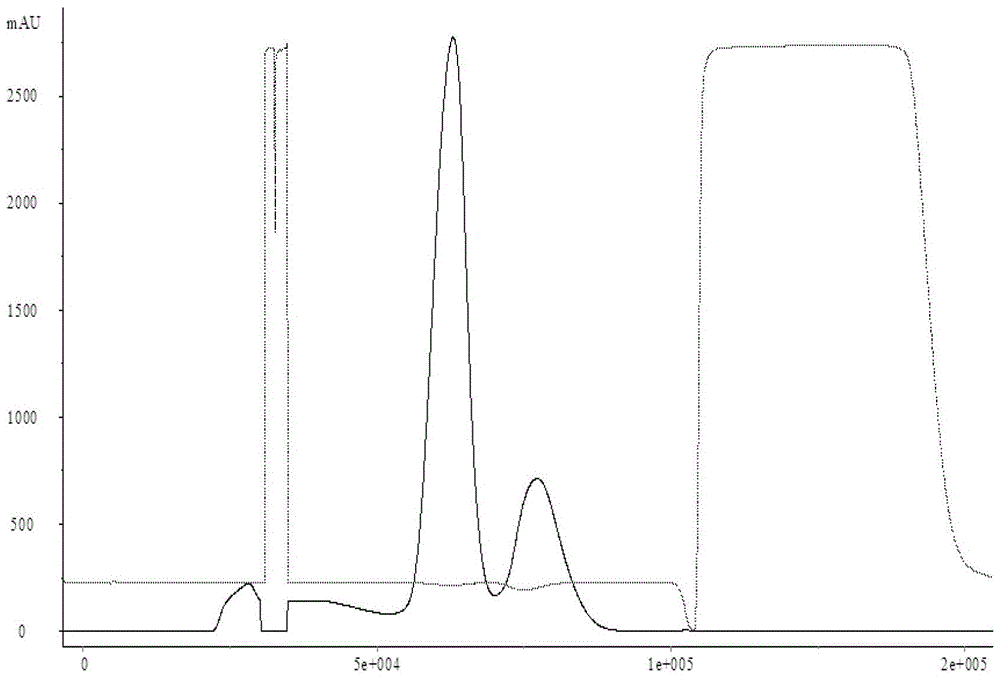
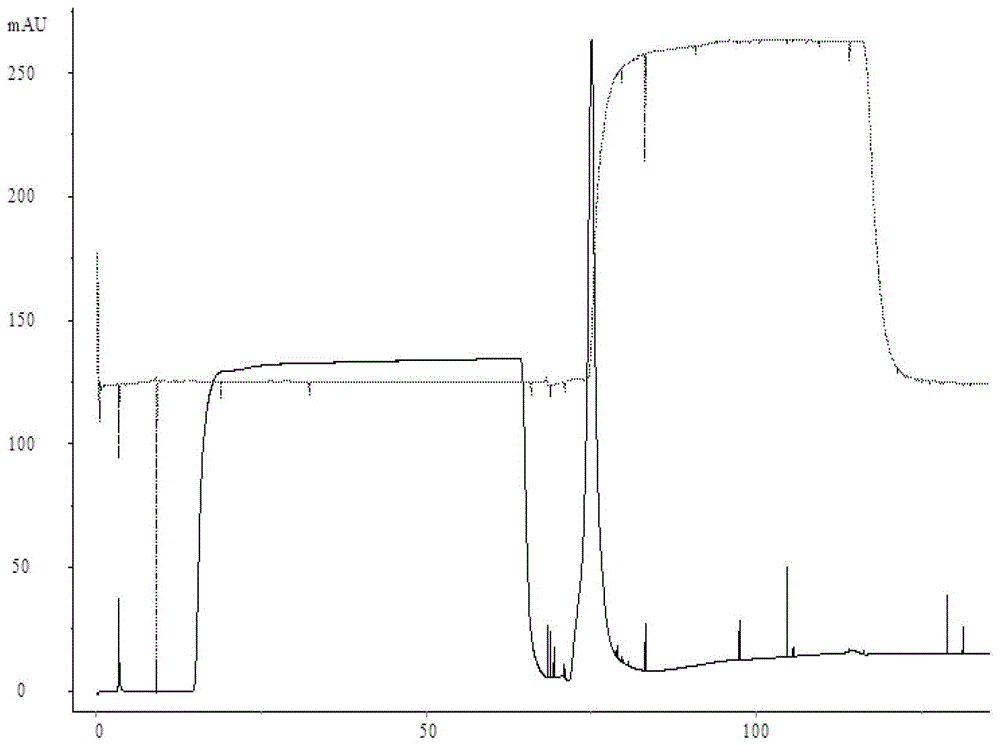
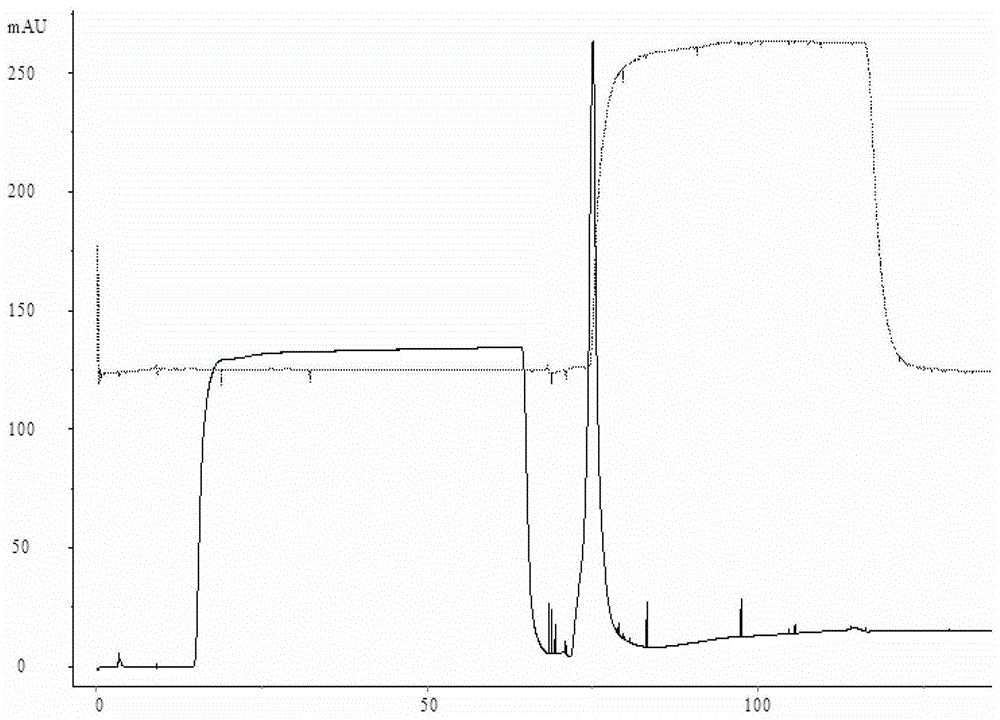
[0026] Use the AKTApilot automatic chromatography equipment to pass 2000mL JE vaccine concentrate (lot number S1) through the BPG200 chromatography column, Sepharose 4Fast Flow gel filtration chromatography, the ultraviolet detection wavelength is 280nm, the flow rate is 6cm / h, and the mobile phase is pH7.2 10mmol / L PBS (NaCl 0.2mol / L), collect the first absorption peak, that is, the preliminary purification solution of Japanese encephalitis virus protein (batch number S2). Then use 10mmol / L PBS (NaCl0.2mol / L) with pH 7.2 to equilibrate the DEAE Sepharose Fast Flow chromatography column (column bed volume 1000mL) to UV and conductance balance, flow rate 3cm / h. Then the preliminary purified liquid is loaded into the DEAE Sepharose Fast Flow chromatography medium by 2 times column bed volume (2000mL), the flow velocity is 2cm / h, and the host DNA is firmly adsorbed on the DEAE Sepharose Fast Flow chromatography medium, while the Japanese encephalitis virus protein and The binding...
Embodiment 2
[0028] Use AKTApilot automatic chromatography equipment to pass 2000mL JE vaccine concentrate through BPG200 chromatography column, Sepharose 4Fast Flow gel filtration chromatography, UV detection wavelength 280nm, flow rate 6cm / h, mobile phase 10mmol / L PBS with pH 7.4 (NaCl 0.3mol / L), collect the first absorption peak, i.e. the preliminary purified liquid of Japanese encephalitis virus protein. Then equilibrate the QSepharose Fast Flow chromatography column with pH 7.4 10mmol / L PBS (NaCl 0.3mol / L) to reach UV and conductance balance, and flow rate 3cm / h. Then the preliminary purified solution was loaded into the Q Sepharose Fast Flow chromatography medium by 2 times the column bed volume (2000mL), and the flow rate was 2cm / h. The host DNA was firmly adsorbed on the Q Sepharose Fast Flow chromatography medium, while the Japanese encephalitis virus protein The binding site with the ion exchange medium is replaced by the ions in the buffer, directly penetrated, the ultraviolet d...
Embodiment 3
[0030] Use AKTApilot automatic chromatography equipment to pass 2000mL JE vaccine concentrate through BPG200 chromatography column, Sepharose 4Fast Flow gel filtration chromatography, UV detection wavelength 280nm, flow rate 6cm / h, mobile phase 10mmol / L PBS with pH 7.4 (NaCl 0.4mol / L), collect the first absorption peak, i.e. the preliminary purified liquid of Japanese encephalitis virus protein. Then equilibrate the CaptoQ chromatography column with 10 mmol / L PBS (NaCl 0.4 mol / L) of pH 7.4 until the UV and conductance are balanced, and the flow rate is 3 cm / h. Then the preliminary purified liquid is loaded into the Capto Q chromatography medium by 2 times the column bed volume (2000mL), the flow rate is 2cm / h, the host DNA is firmly adsorbed on the Capto Q chromatography medium, and the JE virus protein and the ion exchange medium The binding site is replaced by ions in the buffer, directly penetrated, the UV detection wavelength is 280nm, and the absorption peak is collected....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com