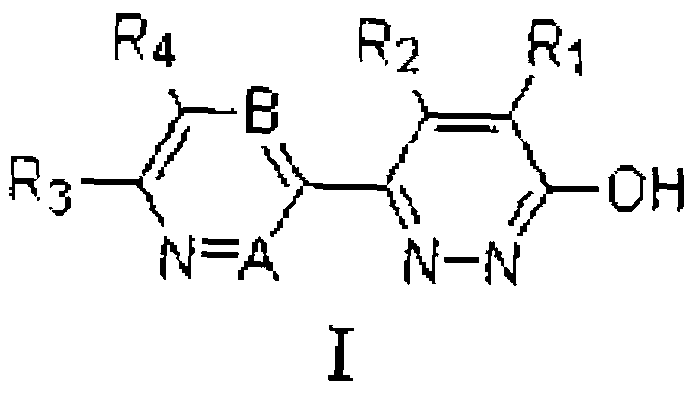
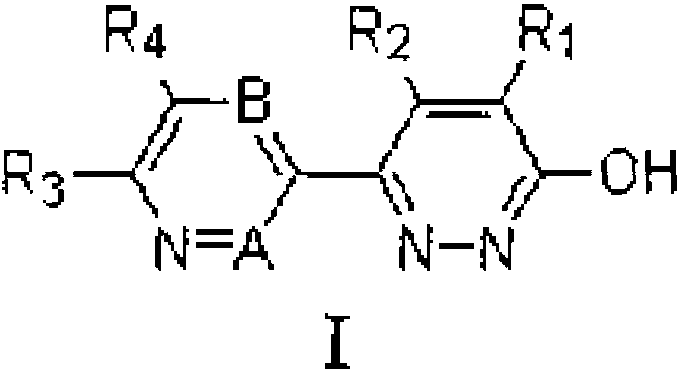
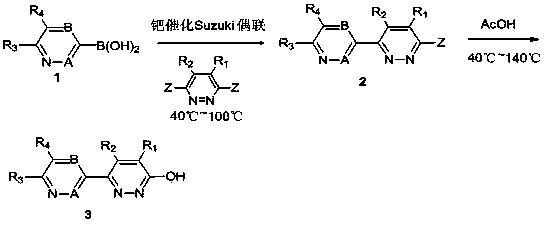
Pyridazinone derivatives and preparation method and application thereof
A technology for pyridazinone and derivatives is applied in the field of compounds and their preparation, and achieves the effects of simple synthesis method and obvious anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Intermediate 5a Synthesis
[0044]
[0045] Compound ( 4a) (200mg, 0.7245mmol) was mixed with 5ml AcOH and refluxed at an external temperature of 120°C for 2h. TLC detection (PE: EA = 5: 1), the reaction of the raw materials was complete, and the reaction was stopped. Pour the reaction solution into water, adjust the pH to neutral with ammonia water, extract the reaction solution with EA, combine the organic layers, wash with saturated brine, and anhydrous Na 2 SO 4 Dried to obtain 160mg of light yellow solid, namely 5a , yield 85%. m.p241-243°C. 1 HNMR {400 MHz, DMSO-d6 (TMS), δ (ppm)}: 13.507(s, 1H), 9.164(d, 1H, J =2.0), 8.649 (d, 1H, J =2.4), 8.231 (d, 1H, J =10.0), 7.090(d, 1H, J =10.0).
Embodiment 2
[0046] Example 2 compound 7a Synthesis
[0047]
[0048] Compound ( 4a ) (100mg, 0.362mmol) was mixed with 5ml AcOH and refluxed at an external temperature of 120°C for 20h. TLC detection (PE:EA=3:1), the reaction of the raw material was complete, and the reaction was stopped. Pour the reaction solution into water, adjust the pH to neutral with ammonia water, extract the reaction solution with EA, combine the organic layers, wash with saturated brine, and anhydrous Na 2 SO 4 Dried to obtain 60mg of light yellow solid, namely 7a , yield 68.6%.
Embodiment 3
[0049] Example 3 Compound 7b Synthesis
[0050]
[0051] Compound ( 5a ) (50mg, 0.194mmol) and piperidine (2ml) were refluxed at an external temperature of 80°C for 1h, TLC detection (PE:Acetone=1:1) The reaction of the raw materials was complete. Spin to dry piperidine, dissolve the reaction solution with EA, apply the sample by dry method, and perform column chromatography (PA: Acetone = 10:1) to obtain a dark yellow solid. that is 7b . Yield: 85%. m.p:168~170℃. 1 HNMR {400 MHz, CDCl 3 (TMS), δ (ppm)}: 10.916 (s,1H); 8.656 (s,1H); 8.249(s,1H); 7.6855 (d, 1H, J =10.0); 7.062 (d, 1H, J =9.6); 3.662 (s, 4H); 1.985 (s, 4H): 1.597 (s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com