Lansoprazole compound
A technology of lansoprazole and lansoprazole crystals, which is applied in the field of chemical drugs, can solve the problems of not showing anticholinergic or histamine H2 receptor characteristics, and achieve stable properties, short production cycle and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Lansoprazole crystal form and its preparation
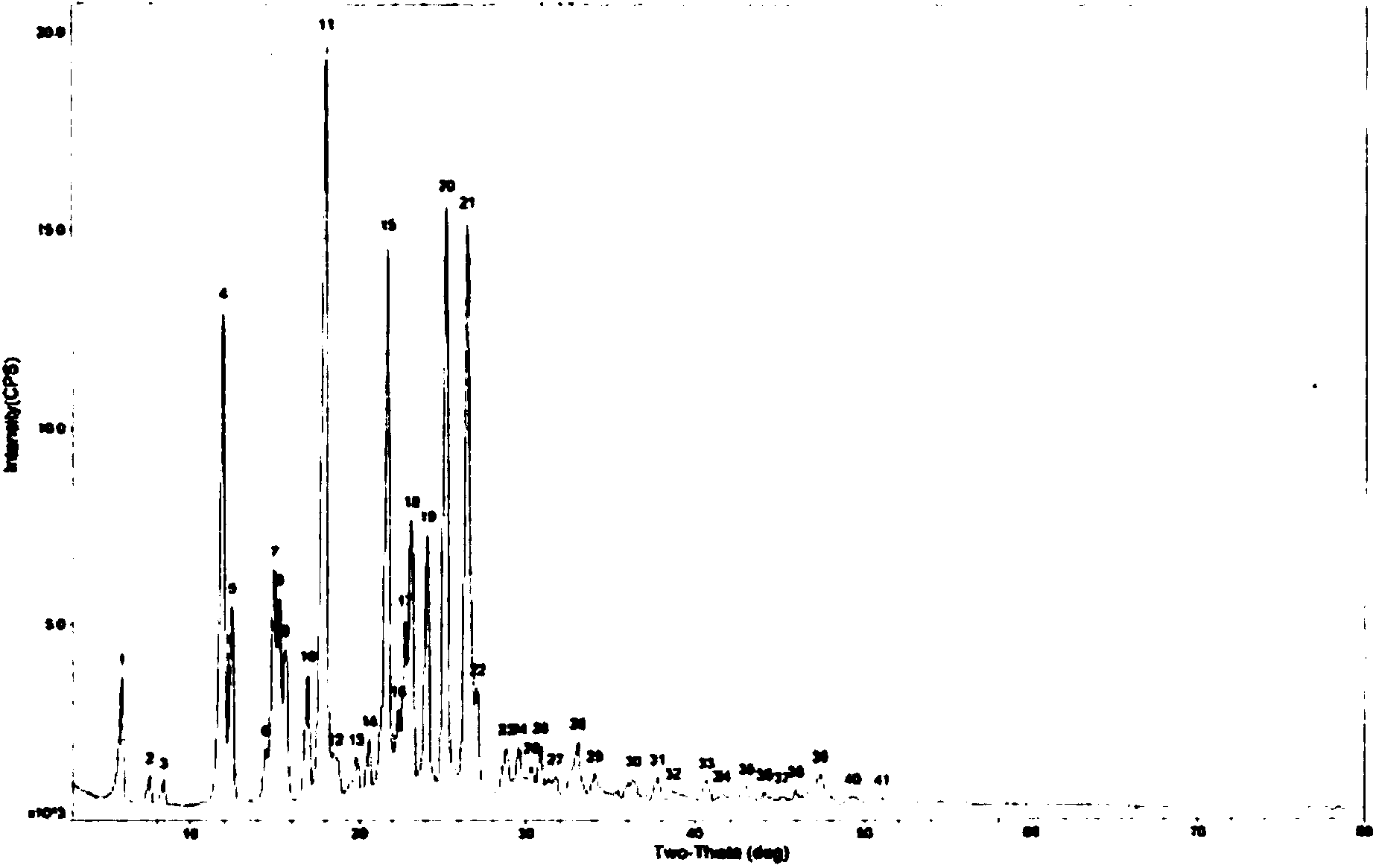
[0032] Dissolve 10 g of lansoprazole in 26 mL of isobutanol: ammonia (volume ratio v / v of 25:1) solution and stir for 1.5 hours; filter with suction to remove insoluble solid particles that may exist; cool the solution to room temperature; Placed in an open container, crystals gradually precipitate out of the solution; collect the obtained crystal particles and vacuum-dry to constant weight at 60°C to obtain 9.45 g of lansoprazole crystal form of the present invention. The X-ray powder diffraction data is shown in figure 1.
Embodiment 2
[0033] Example 2 Lansoprazole crystal form and its preparation
[0034] Dissolve 16 g of lansoprazole in 54 mL of isobutanol: ammonia (volume ratio v / v: 25: 2) solution and stir for 1.5 hours; filter with suction to remove insoluble solid particles that may exist; cool the solution to room temperature; Placed in an open container, crystals gradually precipitate out of the solution; the obtained crystal particles are collected and dried in vacuum at 60°C to a constant weight to obtain 14.96g of lansoprazole crystal form of the present invention. Its X-ray powder diffraction data is consistent with Figure 1 is similar.
[0035] Example 2. Lansoprazole enteric-coated tablets and preparation method thereof
[0036] We used mannitol as the main dispersion, modified the prescription and preparation process, and formulated the following prescription, which achieved the expected effect. The prescription is as follows:
[0037]
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com