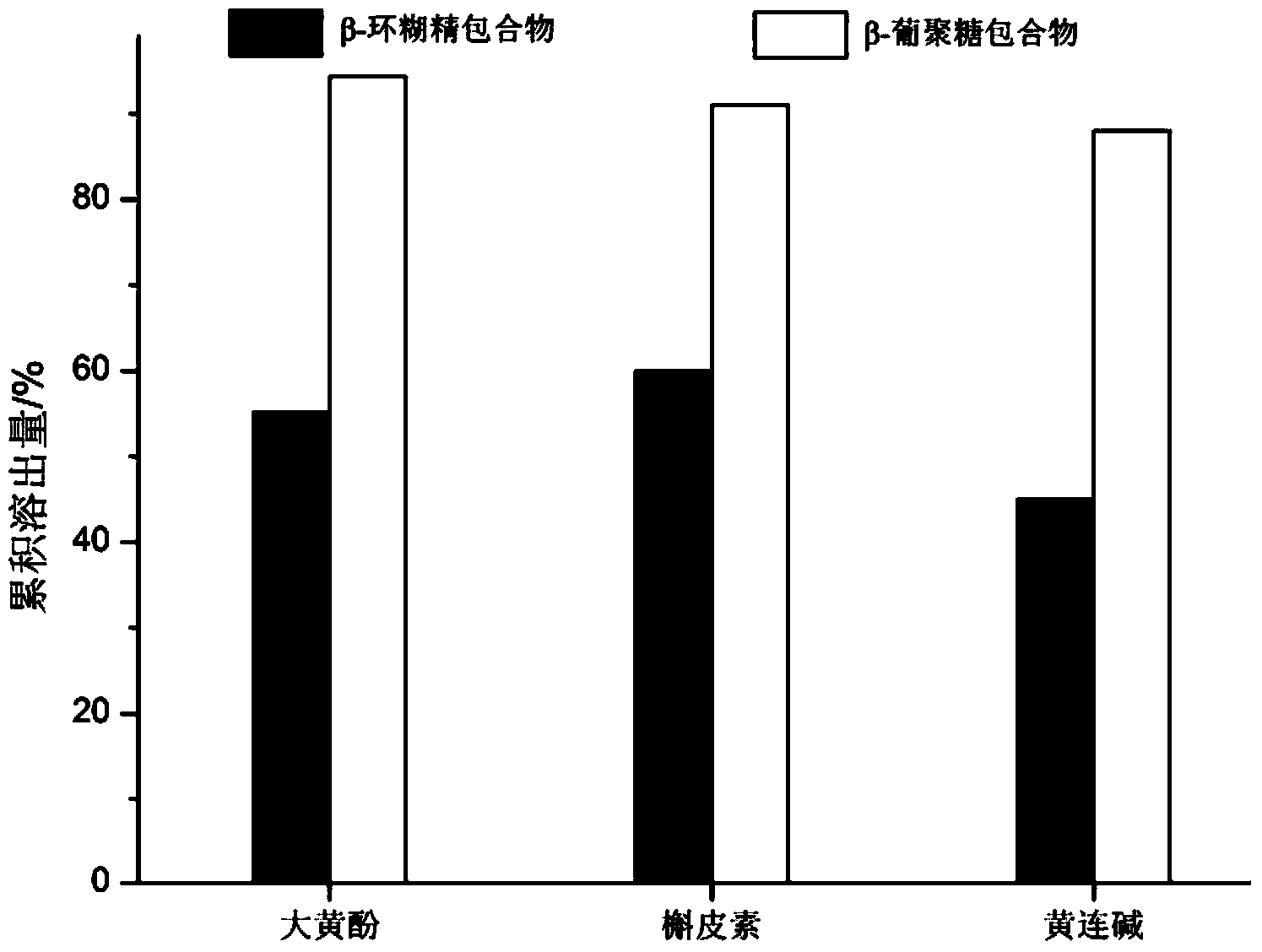
Glucan inclusion compound of water-insoluble small molecule drug and preparation method thereof
A water-insoluble and dextran technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as low bioavailability and poor inclusion effect, and achieve Improve the solubility and solubilization, the preparation method is simple, and the effect of reducing stomach irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of chrysophanol-β-glucan inclusion compound
[0037] Add 1 g of chrysophanol to 3.0 mL of ethanol, add water to dilute to 10 mL, and obtain a clear chrysophanol solution. Under the condition of stirring and heating, completely dissolve the β-glucan, adjust the pH to 6.5, and add the chrysophanol solution to the dextran solution at 80°C, keep it for 30 minutes, and then evaporate the organic solution to obtain the aqueous solution. compound. After the synthesis reaction between chrysophanol and β-glucan, cool down, centrifuge at 14000r / min in a tube centrifuge to remove insoluble matter, ultrafilter with an ultrafiltration membrane with a molecular weight cut-off of 50000Dalton, and vacuum dry or spray dry at 60°C. Chrysophanol-β-glucan clathrate was obtained.
Embodiment 2
[0038] Example 2 Preparation of quercetin-β-glucan inclusion compound
[0039] Take 0.9g of quercetin, add 2mL of ethyl acetate, add water to dilute to 8mL, and stir for 10min to obtain a clear quercetin solution. Under the condition of stirring and heating, completely dissolve the β-glucan, adjust the pH to 5.5, and add the quercetin solution to the glucan solution at 90°C, keep it for 10 minutes, and then evaporate the organic solution to obtain the aqueous solution. clathrate. After the synthetic reaction between quercetin and β-glucan, cool down, centrifuge at 10,000r / min in a tube centrifuge, remove insoluble matter, ultrafilter with an ultrafiltration membrane with a molecular weight cut-off of 6000Dalton, freeze-dry or spray-dry to obtain quercetin Vein-β-glucan inclusion complex.
Embodiment 3
[0040] Example 3 Preparation of Coptisine-β-glucan Inclusion Compound
[0041] Take 0.9g coptisine, add 2mL dimethyl sulfoxide, add water to dilute to 8mL, stir for 10min to obtain a clear solution of coptisine. Under the condition of stirring and heating, completely dissolve the β-glucan, adjust the pH to 10, and the temperature is at 50°C, add coptisine solution to the dextran solution, and keep it for 100 minutes, after which the organic solution is evaporated to obtain an aqueous solution. compound. After the synthetic reaction of coptisine and β-glucan, cool down, centrifuge at 10,000r / min in a tube centrifuge to remove insoluble matter, ultrafilter with an ultrafiltration membrane with a molecular weight cut-off of 15,000Dalton, and freeze-dry or spray-dry to obtain coptisine- β-glucan inclusion complex.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com