Orexin-A pegylation modifier and preparation method thereof
A PEGylation and polyethylene glycol technology, applied in the field of medicinal chemistry, can solve the problems of restricting the development and application of orexin-A, instability of orexin-A, etc., and achieve the effects of good product uniformity and simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation method of orexin-A pegylated modification, comprises the steps:
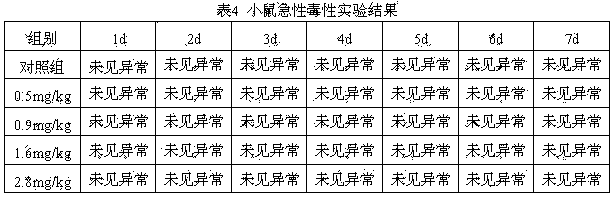
[0019] (1) Weigh 10mg of orexin-A, dissolve it in 10ml of PB buffer (10mM, pH=6), and then add monooxypolyethylene glycol propionaldehyde (molecular weight: 5000Da) at a molar ratio of 1:10 and mix well, and place it at 25°C for 6 hours to terminate the reaction;
[0020] (2) Pass the reaction solution in step (1) through a SepHadex G-25 column to desalt, load the protein collected after desalting onto a QAE-SepHadex A-50 anion exchange column, and elute with 10mM PB buffer, The eluted peaks were collected, concentrated and freeze-dried to obtain the orexin-A PEGylated product.
Embodiment 2
[0022] The preparation method of orexin-A pegylated modification, comprises the steps:
[0023] (1) Weigh 10mg of orexin-A, dissolve it in 10ml of PB buffer (10mM, pH=7), and then add monooxypolyethylene glycol propionaldehyde (molecular weight: 5000Da) at a molar ratio of 1:20 and mix well, place it at 25°C for 5 hours and then terminate the reaction;
[0024] (2) Pass the reaction solution in step (1) through a SepHadex G-25 column to desalt, load the protein collected after desalting onto a QAE-SepHadex A-50 anion exchange column, and elute with 10mM PB buffer, The eluted peaks were collected, concentrated and freeze-dried to obtain the orexin-A PEGylated product.
Embodiment 3
[0026] The preparation method of orexin-A pegylated modification, comprises the steps:
[0027] (1) Weigh 10mg of orexin-A, dissolve it in 10ml of phosphate buffer (10mM, PH=8), and then add monooxypolyethylene glycol propionaldehyde (molecular weight: 5000Da) at a molar ratio of 1:30 ) and mix well, and stop the reaction after reacting in the dark at 25°C for 4 hours;
[0028] (2) Pass the reaction solution in step (1) through a SepHadex G-25 column for desalting, then load the protein collected after desalting onto a QAE-SepHadex A-50 anion exchange column, and elute with 10 mM PB buffer , collect the eluted peaks, concentrate and freeze-dry to obtain the orexin-A PEGylated product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com