Preparation method of levo-oxiracetam lyophilized powder
A freeze-dried powder and drying technology, which is applied in the field of preparation of levooxiracetam freeze-dried powder, can solve the problems of poor product uniformity, inconsistent properties of upper and lower layers, poor product stability, etc. Consistent layer properties and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
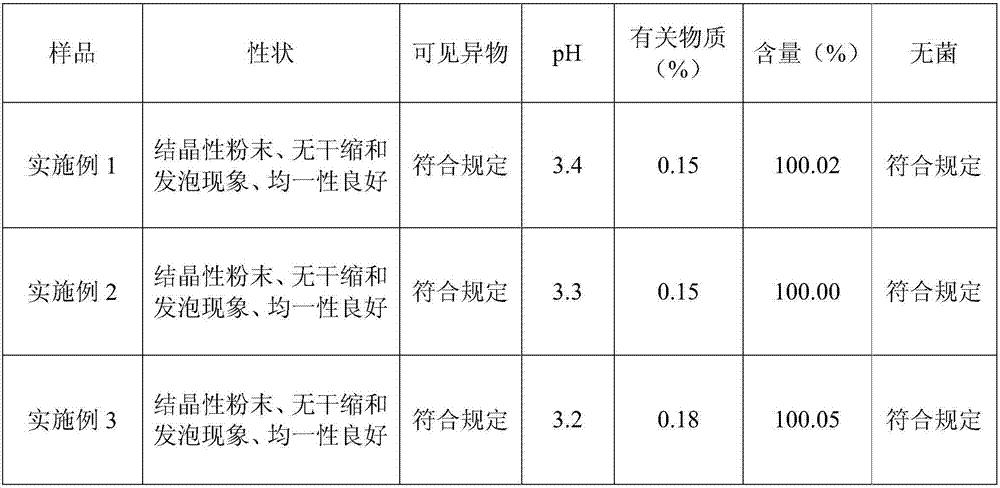
Embodiment 1
[0015] The prescription of the levoxiracetam lyophilized powder of embodiment 1 is shown in the table below:
[0016] prescription
weight percentage
Levoxiracetam
50%
L-serine
25%
14.9%
polyethylene glycol 2000
5%
5%
0.1%
[0017] The preparation method of the levoxiracetam freeze-dried powder of embodiment 1 may further comprise the steps:
[0018] (1) Concentrated preparation: put the raw and auxiliary materials of the prescribed amount in a container, add sterilized water for injection with 10 times the weight of levoxiracetam and stir, after dissolving, add activated carbon for needles with a mass fraction of 0.1%, and stir for 30 minutes. Then filter with a 0.45 micron microporous membrane, collect the filtrate, and set aside;
[0019] (2) Dilute preparation: add sterile water for injection to the filtrate to 1000 times the volume of the filtrate, adjust the p...
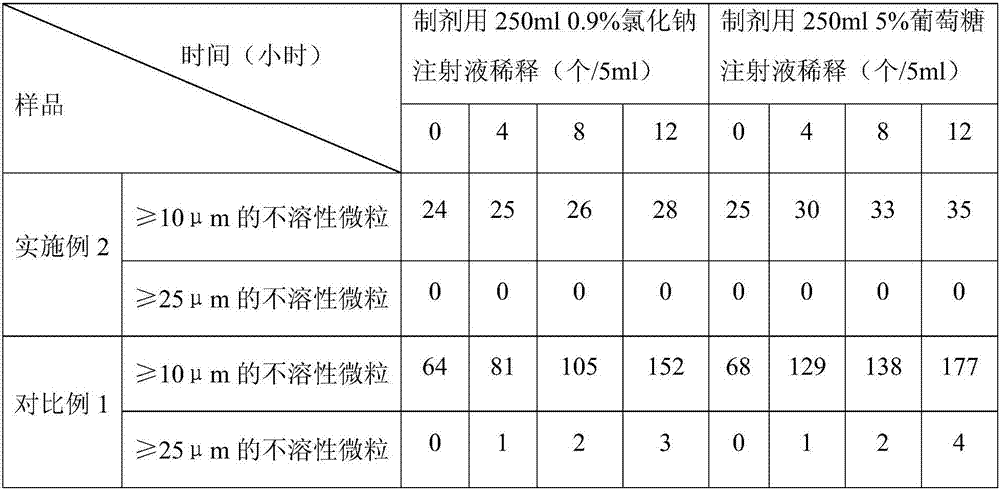
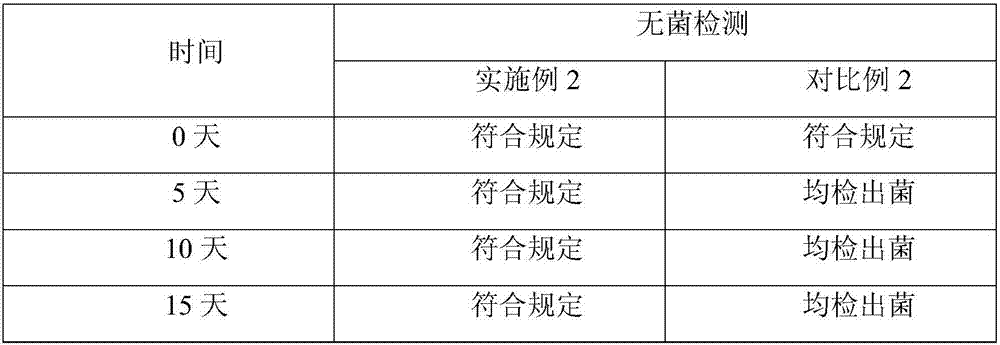
Embodiment 2
[0023] The prescription of the levoxiracetam lyophilized powder of embodiment 2 is shown in the table below:
[0024] prescription
[0025] The preparation method of the levoxiracetam freeze-dried powder of embodiment 2 is the same as that of embodiment 1.
Embodiment 3
[0027] The prescription of the levoxiracetam freeze-dried powder of embodiment 3 is shown in the table below:
[0028] prescription
[0029] The preparation method of the levoxiracetam freeze-dried powder of embodiment 3 is the same as that of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com