Application of cytochalasin H in preparation of Parkinson's disease resistant drugs
A Parkinson's disease compound technology, applied in the application field of the cytochalasin compound cytochalasin H in the preparation of anti-Parkinson's disease drugs, can solve the problems that the application of cytochalasin H has not yet been discovered, and achieve low effective drug concentration and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
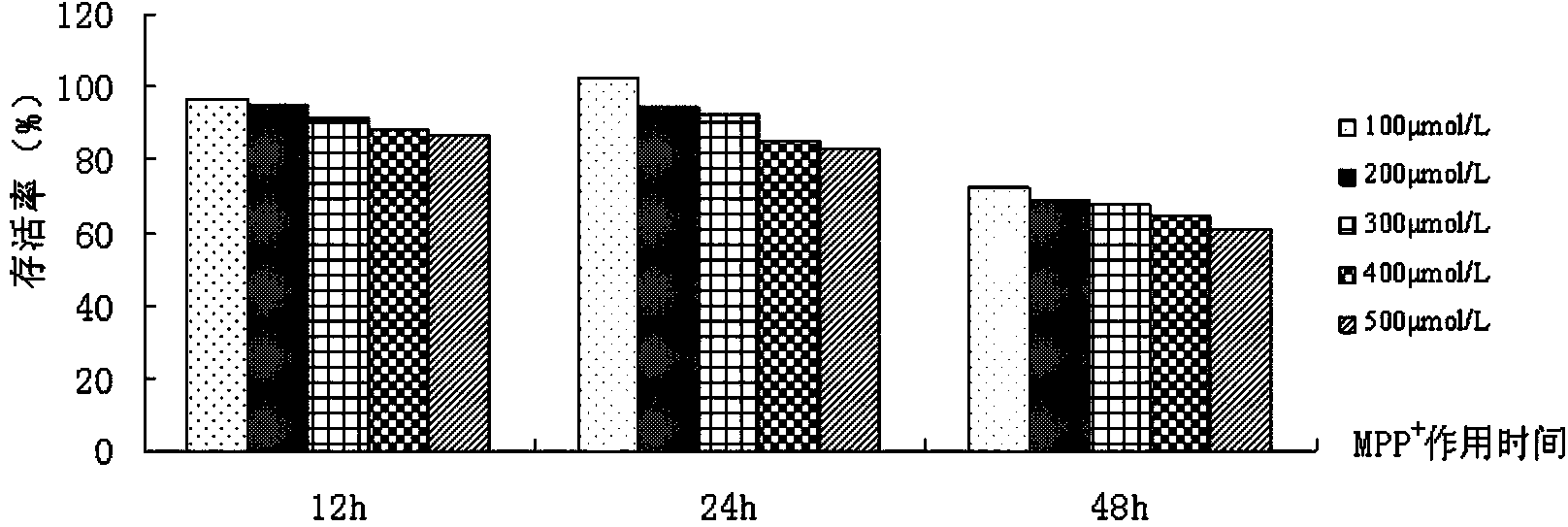
[0025] Example 1.MPP + Replicate the dose-effect and time-effect relationship of PD cell model
[0026] Experimental instruments and materials:
[0027] PB11OS electronic balance (Sartorius, Germany);
[0028] SW-CJ-1F ultra-clean bench (Suzhou Purification Equipment Co., Ltd.);
[0029] Forma3111 water-jacketed carbon dioxide incubator (Thermo / USA);
[0030] ELX-800UV microplate reader (BIO-TEK, USA);
[0031] Rat pheochromocytoma PCl2 cells (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences);
[0032] Newborn bovine serum (Hangzhou Sijiqing Bioengineering Materials Co., Ltd.);
[0033] DMEM (GIBCO Corporation);
[0034] MPP + (SIGMA-Aldrich);
[0035] MTT (AMROSCO Corporation);
[0036] DMSO (AMROSCO Corporation).
[0037] experimental method:
[0038] PC12 cells were cultured in DMEM medium containing 10% newborn bovine serum in CO 2 Incubator (37°C, 5% CO 2 , with a relative humidity of 95%), passaging every 3-4 days. Take the cells in...
Embodiment 2
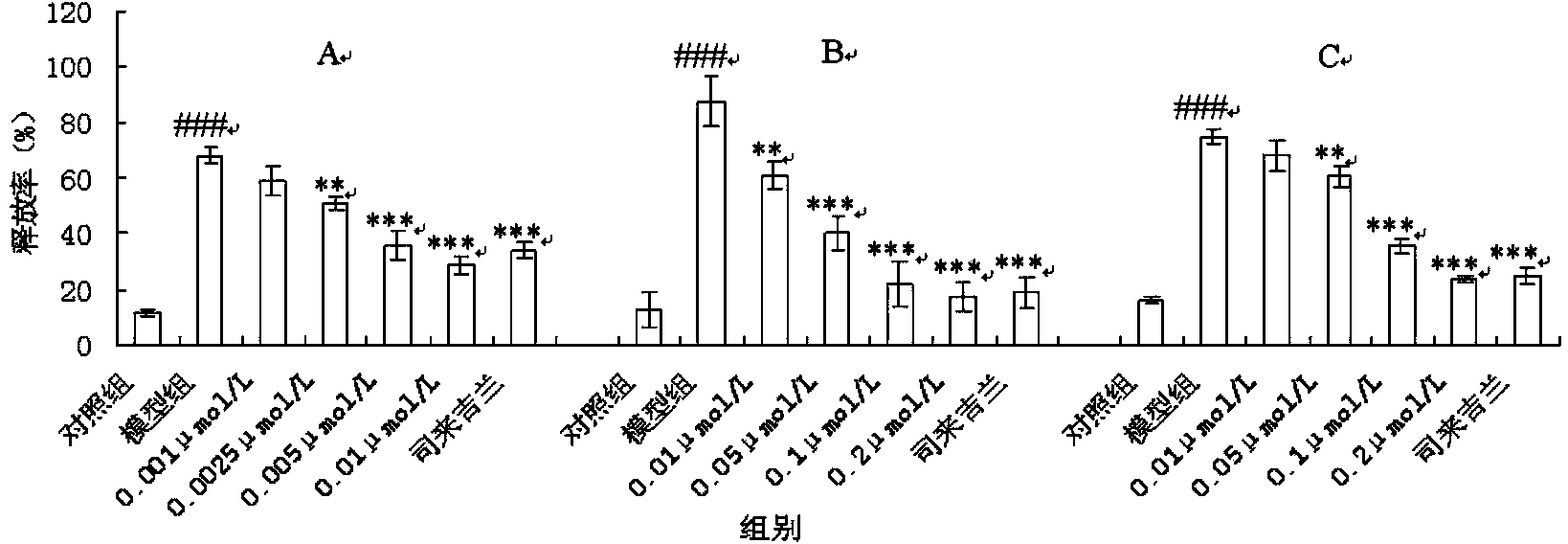
[0041] Example 2.cytochalasin H to MPP+ Effects of Induced PC12 Cell Injury
[0042] Experimental instruments and materials:
[0043] PB11OS electronic balance (Sartorius, Germany);
[0044] SW-CJ-1F ultra-clean bench (Suzhou Purification Equipment Co., Ltd.);
[0045] Forma3111 water-jacketed carbon dioxide incubator (Thermo / USA);
[0046] ELX-800UV microplate reader (BIO-TEK, USA);
[0047] Rat pheochromocytoma PCl2 cells (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences);
[0048] Cytochalasin H (can be purchased from Santa Cruz Biotechnology or Sigma-Aldrich; it can also be isolated from the solid fermentation product of endophyte Phomopsis sp. IFB-E060 (GenBank No.: GU989315). Isolation and identification of endophytic strain IFB-E060 And the extraction, separation and structural identification of fermentation and compounds can be carried out with reference to the literature [Xu S, et al. Chem Biodivers2009, 6(5):739-745]);
[0049] Newborn bo...
Embodiment 3
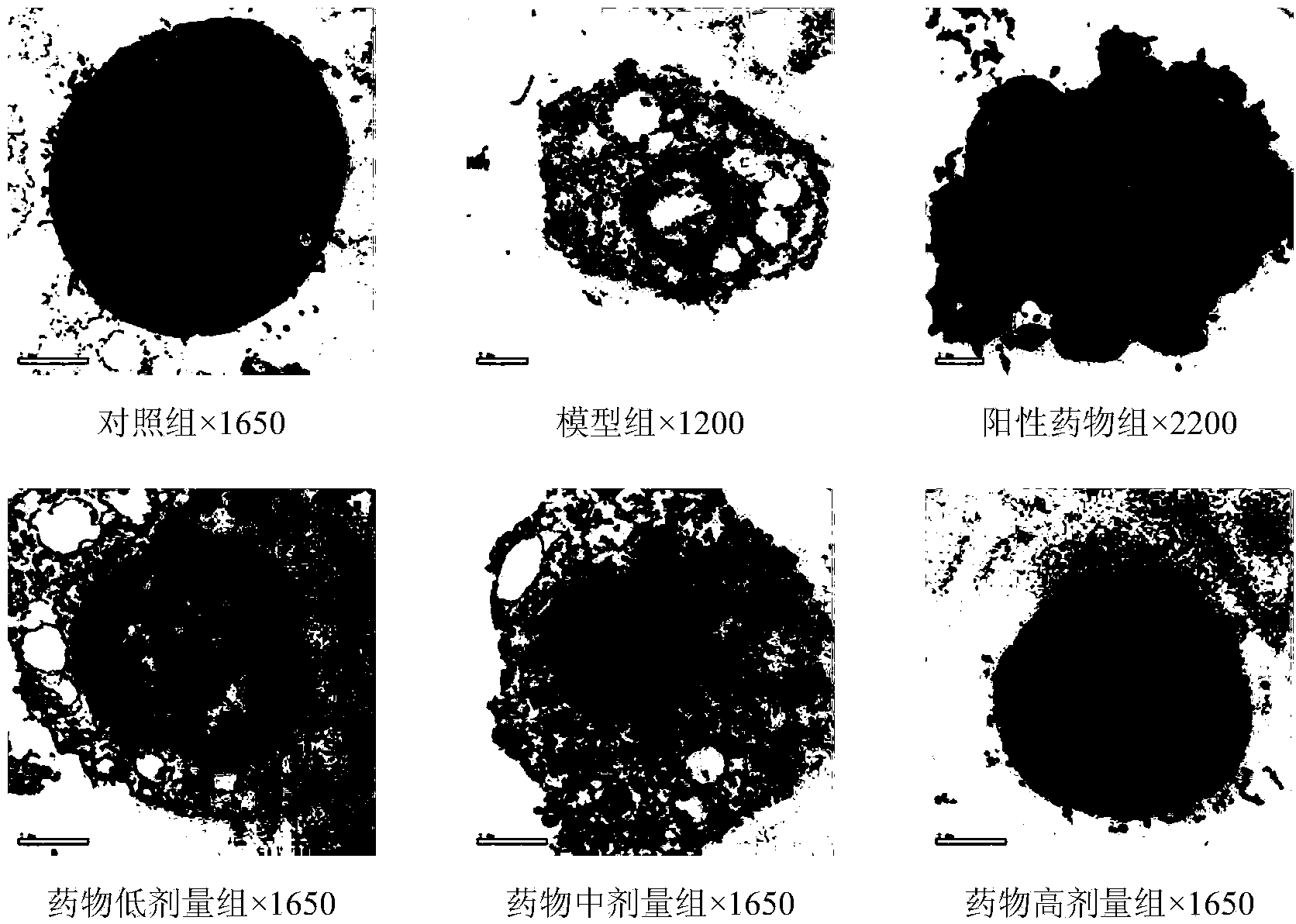
[0071] Example 3.cytochalasin H to MPP + Effect of Inducing PC12 Cell Apoptosis
[0072] Experimental instruments and materials:
[0073] PB11OS electronic balance (Sartorius, Germany);
[0074] SW-CJ-1F ultra-clean bench (Suzhou Purification Equipment Co., Ltd.);
[0075] OPTIPHOT-2 fluorescence microscope (NIKON, Japan);
[0076] XDS-1B inverted biological microscope (Chongqing Optical Instrument Co., Ltd.);
[0077] Forma3111 water-jacketed carbon dioxide incubator (Thermo / USA);
[0078] Flow cytometry (FACSAria, BD, USA);
[0079] Rat pheochromocytoma PCl2 cells (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences);
[0080] cytochalasin H (same as Example 2);
[0081] DMSO (AMROSCO Corporation);
[0082] Newborn bovine serum (Hangzhou Sijiqing Bioengineering Materials Co., Ltd.);
[0083] DMEM (GIBCO Corporation);
[0084] MPP + (SIGMA-Aldrich);
[0085] R-(-)-Deprenyl HCl (Enzo Life Sciences, Inc.);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com